Fabrication of Engineered Vascular Flaps Using 3D Printing Technologies
Summary
Engineered flaps require an incorporated functional vascular network. In this protocol, we present a method of fabricating a 3D printed tissue flap containing a hierarchical vascular network and its direct microsurgical anastomoses to rat femoral artery.
Abstract
Engineering implantable, functional, thick tissues requires designing a hierarchical vascular network. 3D bioprinting is a technology used to create tissues by adding layer upon layer of printable biomaterials, termed bioinks, and cells in an orderly and automatic manner, which allows for creating highly intricate structures that traditional tissue engineering techniques cannot achieve. Thus, 3D bioprinting is an appealing in vitro approach to mimic the native vasculature complex structure, ranging from millimetric vessels to microvascular networks.
Advances in 3D bioprinting in granular hydrogels enabled the high-resolution extrusion of low-viscosity extracellular matrix-based bioinks. This work presents a combined 3D bioprinting and sacrificial mold-based 3D printing approach for fabricating engineered vascularized tissue flaps. 3D bioprinting of endothelial and support cells using recombinant collagen-methacrylate bioink within a gelatin support bath is utilized for the fabrication of a self-assembled capillary network. This printed microvasculature is assembled around a mesoscale vessel-like porous scaffold, fabricated using a sacrificial 3D printed mold, and is seeded with endothelial cells.
This assembly induces the endothelium of the mesoscale vessel to anastomose with the surrounding capillary network, establishing a hierarchical vascular network within an engineered tissue flap. The engineered flap is then directly implanted by surgical anastomosis to a rat femoral artery using a cuff technique. The described methods can be expanded for the fabrication of various vascularized tissue flaps for use in reconstruction surgery and vascularization studies.
Introduction
Severe tissue defects are caused by traumatic injuries, congenital defects, or disease, and the current gold standard for treating these defects is by using autologous grafts, vascularized tissue flaps, and microvascular free flaps as tissue substitutes. However, these options have the drawbacks of limited donor site tissue and donor site morbidity1. Thus, there is a growing demand for alternative tissue substitutes that can be used to correct these defects2. The thickness of the engineered tissue constructs is limited by the diffusion of nutrients and gases toward the cells, and, therefore, a proper vascular network is essential for generating large, thick, and properly nourished scaffolds.
Several approaches have been applied to promote the vascularization of engineered implants3, including the in vivo recruitment of vascular support from the host, the delivery of growth factors and cytokines within the scaffolds, the prevascularization of implants, the generation of a perfusable branching microvessel bed using micropatterning techniques4, the use of sacrificial materials for vascular channel/network formation5, as well as the creation of channels within 3D bioprinted constructs5,6. Vascularization of thick tissues requires the incorporation of a hierarchical vascular network consisting of macro-scale and microcapillary-scale vessels. The macro-scale vessels distribute blood effectively throughout the construct and allow for microsurgical anastomoses with the host blood vessels, while the microcapillary-scale vessels allow for nutrient diffusion.
Bioprinting has gained strong attention in recent years due to the advantages it offers over conventional tissue engineering methods. Tissues and organs are complex and intricate 3D objects with a specific architecture. 3D bioprinting, with its ability to deposit layers of biomaterials in high resolution, enables the capability of creating complex tissue and organ substitutes (e.g., kidney, lung, liver)7. Several printing technologies have been adapted for bioprinting, including extrusion-based, inkjet8, laser-assisted deposition9,10, and stereolithography-based11,12 bioprinting. The extrusion-based technologies rely on extruding the material through a nozzle by applying pressure on the material bulk surface opposite to the nozzle.
Free-form reversible embedding of suspended hydrogels (FRESH) is a bioprinting technique13,14 that uses a granular support material in which the extruded material is deposited and fixed in place by the support bath. The support bath gives mechanical support for the extruded, pre-crosslinked bioink until its crosslinking. The main advantage of this technique is that the support bath allows for extruding low-viscosity materials that cannot maintain their shape after extrusion and before crosslinking15. This expands the pool of available materials that can be used as bioinks.
This paper presents a protocol for the generation of a vascularized flap that combines microscale and mesoscale vasculatures. To achieve this, bioprinted, self-assembling, microvascular networks are generated in recombinant human collagen methacrylate (rhCollMA) hydrogel, which then connects to the interior of a larger, implantable, vascular scaffold, resulting in a fully engineered tissue flap16. To establish rapid and direct perfusion of engineered tissues, a direct microsurgical anastomosis to host vessels is required. The vascular scaffold does not have sufficient suture retention strength to be anastomosed using traditional microsurgical vessel wall suturing. Therefore, we describe a "cuff"17,18,19 method to achieve an anastomosis with a rat's common femoral artery. In this method, the vessel ends are secured with circumferential sutures, without the need to perforate the vessel wall.
Although the proposed protocol has been prepared to study hierarchical vasculature in the rhCollMA environment, this approach can be expanded and applied to a variety of new applications. The protocol can be applied to bioprinting various tissue-specific cells in different bioinks. Moreover, the geometry and size of the constructs can be easily modified to fit specific requirements, such as large tissue reconstruction or biological studies.
Protocol
All animal procedures were approved and conducted under the supervision of the Technion Pre-Clinical Research Authority (PCRA Technion, ethics approval no. 058-05-20). Male Sprague-Dawley rats (275-350 g) were used for these studies. See the Table of Materials for details related to all materials, equipment, and software used in this protocol.
1. Vascular scaffold fabrication
- Create a 3D computer model of the desired vascular scaffold shape using computer-aided design (CAD) software or download a 3D file of a desired vascular structure from online repositories.
- Create a cylinder with an inner diameter of 0.9 mm, an outer diameter of 2 mm, and a height of 18 mm. Add radial fenestrations with a diameter of 0.22 mm alongside the cylinder wall.
- Create a mold for the scaffold using the 3D design software and export it as an .STL file. Next, add a funnel to the design to ease the filling of the mold later.
- Import the .STL file into the slicing software for the fused deposition modeling (FDM) 3D printer. Slice the model with a layer height of 0.1 mm, and export it as .gcode or send it directly to the 3D printer.
- 3D print the mold on an FDM 3D printer equipped with a 0.25 mm nozzle, using water-soluble butene-diol vinyl alcohol copolymer (BVOH) filament. Store the printed molds in a vacuum chamber until use.
CAUTION: Avoid handling the BVOH filament with bare hands as it is moisture-sensitive. In addition, it is recommended to store the filament in a vacuum chamber to avoid exposure to moisture. - Prepare a polymer solution of a 1:1 mixture of poly-L-lactic-acid (PLLA) and polylactic-co-glycolic-acid (PLGA) in 1,4-dioxane.
- Weigh 350 mg of PLLA and 350 mg of PLGA and transfer to a 20 mL glass vial.
- Measure 10 mL of 1,4-dioxane and transfer to the glass vial using a glass pipette. Add a small magnetic stir bar to the glass vial.
CAUTION: 1,4-Dioxane is a hazardous organic solvent. Use appropriate personal safety equipment and work inside a fume hood. - Place the glass vial inside a hot water bath heated to 70 °C and mix the contents overnight until all the polymers dissolve fully. Store the solution in an airtight glass container until use.
- Fill the BVOH molds with the PLLA:PLGA solution.
- Using a positive displacement pipette, measure 30 µL of the polymer solution and fill in the mold.
NOTE: The volume needed to fill the mold will change according to the volume of the designed vessel. Keep adding the polymer solution until the funnel of the mold is entirely filled. - Centrifuge the mold at 100 × g for 2 min. Top up the mold with a further 20 µL of the polymer solution to ensure complete filling.
- Using a positive displacement pipette, measure 30 µL of the polymer solution and fill in the mold.
- Freeze the filled molds at -80 °C for at least 30 min to ensure all the polymer solution freezes. Remove the solvent from the molds by lyophilizing them overnight.
NOTE: The color of the polymer in the mold should turn from clear to white after the freeze-drying process. - To remove the sacrificial mold material, transfer the molds after the freeze-drying process to a 5 L deionized water bath under gentle stirring. Replace the water in the bath when it gets cloudy. When all the BVOH dissolves, air-dry the scaffolds and store them in a vacuum chamber until use.
2. Coating the vascular scaffold with fibronectin
- Disinfect the PLLA:PLGA vascular scaffolds by immersing them in 70% ethanol for 30 min.
- Wash the scaffolds 3x 5 min with PBS.
- Prepare a dilution of 50 µg/mL human fibronectin in PBS and coat the scaffolds.
- Mix 500 µL of stock fibronectin solution (1 mg/mL) with 9.5 mL of PBS.
- Immerse the disinfected vascular scaffolds in this fibronectin solution and incubate them at 37 °C for 60 min to allow for protein adsorption.
- After incubation, rinse the scaffolds with PBS to remove the unbound fibronectin. Store these coated scaffolds at 4 °C for up to 1 week.
3. rhCollMA bioink preparation
- Prepare a phosphate buffer for neutralizing the acidic rhCollMA bioink stock.
- Prepare a 10x stock of phosphate buffer by dissolving 5.495 g of Na2HPO4, 1.55 g of NaH2PO4, and 30 mg of NaCl in 50 mL of deionized water.
- Prepare a 10-fold dilution of the 10x stock phosphate buffer by combining 1 part of 10x phosphate buffer with 9 parts of deionized water.
- Combine 900 µL of stock rhCollMA solution with 100 µL of 10x phosphate buffer to neutralize the acidic rhCollMA solution.
- Dilute the neutralized rhCollMA solution to the desired concentration of 10 mg/mL by mixing it with the required amount of 1x phosphate buffer.
NOTE: The stock concentration of rhCollMA varies between lots. Therefore, the volume of 1x phosphate buffer needed to reach the desired concentration will vary. - Prepare the porogen-photoinitiator solution (PEO-LAP) to be combined with the diluted neutral rhCollMA.
- Prepare a 1.6% (w/v) solution of poly(ethylene oxide) (PEO) in phenol red-free DMEM by dissolving 160 mg of PEO in 10 mL of DMEM.
- Add 20 mg of lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) to this solution to obtain 0.2% (w/v) LAP in the solution. From this point, cover the solution with aluminum foil or keep it in a dark place to protect it from light.
- Sterilize the PEO-LAP solution by passing it through a 0.22 µm filter. Store this solution at 4 °C for up to 1 week.
- Prior to bioprinting, mix the diluted neutral rhCollMA solution with the PEO-LAP solution in a 1:1 ratio to obtain the final bioink solution. Use pipetting, rather than a vortex, to mix the solutions while avoiding air bubbles.
- If a lot of bubbles get introduced into the solution, centrifuge it at 2,000 × g for 30 s. After centrifugation, mix the bioink solution again to ensure homogeneity.
4. Granular support bath preparation
- Prepare gelatin granular support material.
- In a biological safety cabinet, divide the contents of one 2 g tube of lyophilized support material into two sterile 50 mL conical tubes, each containing approximately 1 g.
- Add 40 mL of cold (4 °C) phenol-red free DMEM to each tube, and vortex vigorously until all the lyophilized support material dissolves.
- Let the resulting slurry sit at 4 °C to allow the support material to rehydrate.
- Degas the support material in a vacuum chamber for 30 min to reduce bubbles.
- Centrifuge the support material at 2,000 × g for 5 min. Ensure that the material is at the bottom of the tube and the supernatant is clear.
- Aspirate the supernatant, while being careful not to aspirate the support material at the bottom.
NOTE: The support material should not flow while slanting the tube after removing the supernatant. If the material flows, centrifuge it again using the same settings but without adding new supernatant.
- Transfer approximately 4 mL of the prepared, compacted support material into each well of a 12-well plate using a positive displacement pipette. Tap the well plate on a hard surface to force the support material to spread evenly in the well.
NOTE: The minimal recommended volume of support material is 3x the volume of the construct that will be printed in it. - Place the well plate with the support material on a cooled bioprinter stage, or at 4 °C, until its use to prevent the gelatin particles from melting.
5. Incorporation of endothelial cells and support cells with the bioink
- Prepare endothelial cell medium according to the manufacturer's instructions by mixing the basal medium with its corresponding medium kit component, including an antibiotic solution, fetal bovine serum, and endothelial cell growth supplements.
- Prepare dental pulp stem cell medium by combining 500 mL of low-glucose DMEM, 58 mL of fetal bovine serum, 5.8 mL of non-essential amino acids (NEAA), 5.8 mL of glutamine substitute, 5.8 mL of 1 M HEPES, and 5.8 mL of penicillin-streptomycin-nystatin solution.
- Prepare a suspension containing 2 × 106 ZsGreen1-expressing human adipose microvascular endothelial cells (HAMEC-ZsGreen1) and 6 × 106 dental pulp stem cells (DPSCs) in 10 mL of endothelial cell medium.
- Centrifuge the cell suspension at 200 × g for 4 min to obtain a cell pellet. Aspirate the supernatant medium.
- Resuspend the cell pellet in 1 mL of rhCollMA bioink prepared previously (containing PEO-LAP) to obtain a bioink with a total cell concentration of 8 × 106 cells per 1 mL of bioink.
CAUTION: After combining the bioink with the cells, proceed immediately to the next step. If cells are left in the suspension for a long time, the cells will sink to the bottom of the tube, and their viability will deteriorate. For experiments not involving cells, either skip Steps 5.1-5.5, or incorporate fluorescent beads with the bioink instead of cells for enhanced microscopic visualization of the printed constructs. - Transfer the cells-bioink mixture into printing cartridges.
- Fit a 0.22 mm internal diameter needle to a 3 mL amber printing cartridge and place the cartridge in a 50 mL conical tube.
- Transfer 1 mL of the cells-bioink mixture to the printing cartridge by filling it from the top, using a positive displacement pipette to reduce bubbles.
- Install the printing cartridge in the appropriate tool at the bioprinter.
6. Bioprinting microvascular networks using rhCollMA bioink
- Create a 3D CAD design for the bioprinted microvascular network.
- Sketch a 2D pattern of a 4 mm square containing a 2 mm diameter circular channel in its center. Extrude the sketch by 4 mm to obtain a 4 mm x 4 mm x 4 mm cube with a central channel. Export this object as an .STL file.
- Import a 12-well plate .STL template into the solid modeling tab in the bioprinter slicing software and place it in the designated area of the virtual print bed.
- Import the .STL file of the cube shape into the solid modeling tab in the bioprinter slicing software. Click on the use external slicer checkbox | configure slicer under the object properties section. In the popup window, choose a rectilinear pattern for the fill pattern, and type 30% in the fill density. Click accept.
- Place a copy of the cube shape in each desired virtual well by clicking on the cube and moving it using the mouse.
- Create new material settings for rhCollMA bioink in the materials tab of the bioprinter slicing software by clicking the add material button. Choose the material's settings for printing. For pressure, type 2 psi in the corresponding box. For speed, type 20 mm/s in the corresponding box.
NOTE: Each bioink material has its own different printing settings that can be saved in the materials list in the application. - Assign the line width and line height values to 0.24 mm and the acceleration value a 400 mm/s2, by typing these values in the corresponding boxes.
- In the solid modeling tab, click on the cube object, and then click on the rhCollMA material from the materials section to assign it to the cube shape. In the bioassembly tab, click on the send print job button to send the print job to the bioprinter.
- Load the printing cartridge loaded with the cellular bioink into the 3 mL ambient pneumatic dispense tool of the 3D extrusion-based bioprinter.
- Set the print bed temperature to 4 °C by clicking on the cool button under the Control-Heating/Cooling tab in the bioprinter interface screen. Load the plate with the support bath on the printer bed.
NOTE: The material's settings for print pressure and speed might change due to differences in ambient temperature or bioink batch variability. For instance, on colder days, a higher pressure or slower speed is needed to extrude the same amount of material. - In the print tab on the bioprinter interface screen, click on the print job sent in Step 6.7. and click start | go to begin the print job.
- After printing, expose the well-plate to a 405 nm light source with an intensity of 3 mW/cm2 for 30 s to initiate the crosslinking of the rhCollMA bioink.
- After crosslinking, incubate the well plate at 37 °C and 5% CO2 for at least 20 min until all the support bath melts.
- Gently aspirate the liquified support bath and replace it with endothelial cell medium. Incubate the constructs at 37 °C and 5% CO2 until use in further steps.
NOTE: Due to the contraction of the construct following printing, it is recommended to perform the assembly Step 7 on the same day as Step 6.
7. Assembling the bioprinted microvascular network with the vascular scaffold to obtain the engineered vascularized flap
- Immediately after support material removal of the bioprinted microvascular network, place a fibronectin-coated PLLA:PLGA vascular scaffold in the main channel of the bioprinted structure.
- Incubate the assembled constructs at 37 °C and 5% CO2 for 2 days.
- Line the lumen of the vascular scaffold with endothelial cells.
- Prepare a cell suspension of tdTomato-expressing human adipose microvascular endothelial cells (HAMEC-tdTomato) at a concentration of 1 × 107 cells/mL.
- Place a 20 µL droplet of this cell suspension on a hydrophobic surface (i.e., a non-tissue culture [nonTC] 10 cm dish).
- Gently place the vascular scaffolds on top of the droplet, such that the lumen of the scaffold on one end makes contact with the cell droplet.
- Aspirate the droplet from the opposing end of the scaffold (i.e., the end that is not contacting the droplet), filling its lumen with the cell suspension.
- Place the seeded scaffold in a microcentrifuge tube and put it in a rotator inside in a humidified incubator for 60 min. Next, transfer the scaffold into a 12-well plate and add 2 mL of endothelial cell medium.
- Culture the engineered flaps for 7 days, while replacing the medium every 2 days with fresh endothelial cell medium.
8. Confocal microscopy and immunofluorescence staining of the engineered flaps
- After 4 days and 7 days of culture, perform live-cell imaging of the assembled scaffolds using a confocal laser-scanning microscope.
- Define the fluorescence channels for ZsGreen1 and tdTomato fluorescent proteins on the microscope image acquisition software.
- Choose a 5x/0.16 objective lens with 0.5x zoom (pixel size 5 µm), and define a Z-stack with 22 slices with a thickness of 41 µm each.
- Define a 3 x 2 tile scan to image and capture the entire construct.
- Adjust the laser intensity and gain values to obtain a clear fluorescent signal with no saturation and minimal background and acquire the images.
- Perform a maximum-intensity projection of the acquired Z-stack to obtain a single 2D image using the microscope image acquisition software or similar image analysis software.
- Perform higher magnification imaging of a region of interest.
- Switch to a 10x/0.3 objective lens with 0.5 zoom (pixel size 1.25 µm) and define a Z-stack with 9 slices of thicknesses of 41 µm.
- Perform Steps 8.2.-8.3.
- After 7 days of culture, fix the engineered flaps by submerging them in 4% paraformaldehyde for 20 min.
- Wash the constructs 3x 5 min with PBS.
- Add 0.3% (v/v) Triton-X in PBS to the constructs and incubate for 15 min at room temperature to permeabilize the cells.
- Wash the constructs 3x 5 min with PBS.
- Prepare a blocking solution by dissolving 5% (w/v) bovine serum albumin (BSA) in PBS. Add the blocking solution to the engineered flaps and incubate for 1 h at room temperature.
- Prepare the primary antibody solution by diluting mouse anti-smooth muscle actin (anti-SMA) 1:50 antibody in the 5% BSA solution prepared previously to stain the SMA-expressing support cells.
- Incubate the scaffolds in the primary antibody solution overnight at 4 °C.
- Wash 3x 5 min with PBS.
- Prepare the secondary antibody solution by diluting Cy3-conjugated goat anti-mouse IgG 1:400 antibody and 4',6-diamidino-2-phenylindole (DAPI, 2.5 µg/mL) in PBS.
- Apply the secondary antibody solution to the scaffolds and incubate for 3 h at room temperature.
- Wash 3x 5 min with PBS.
- Store the constructs at 4 °C in PBS for up to 1 month.
- Image the stained scaffolds using a confocal laser-scanning microscope.
- Define the three fluorescence channels (DAPI, ZsGreen, and Cy3) on the microscope image acquisition software.
- Define a Z-stack with a section thickness of 2 µm spanning a total thickness of 50 µm. Next, define a tile scan to image larger areas of the construct.
- Adjust the acquisition parameters to obtain a clear fluorescent signal with no saturation and minimal background.
- Acquire the images using 20x/0.8 objective with 1.0x zoom (pixel size 0.31 µm).
- Perform a maximum-intensity projection of the acquired Z-stack to obtain a single 2D image using the microscope image acquisition software or similar image analysis software.
9. Anastomosing the engineered flap directly to a rat's femoral artery using a cuff technique
- Prepare and sterilize (autoclave) the following surgical tools: No. 15 scalpel, a pair of fine-toothed forceps, and a small pair of ring-handled scissors. In addition, prepare and sterilize the following microsurgical tools: a straight fine-pointed No. 3 jeweler's forceps, an angled jeweler's forceps, a round-handled needle holder with curved jaws, a pair of vessel dilators, dissecting scissors, adventitia scissors, as well as vessel clamps with their applicator.
- Prepare a solution of 100 IU/mL heparinized saline by diluting 5 mL of stock heparin solution with 195 mL of saline.
- Cut and sterilize polyimide cuffs.
- Cut 2.5 mm sections of a polyimide tube under a dissecting microscope.
- At one end of each section, make a 1.25 mm lengthwise incision in the wall of the tube to obtain a cuff handle. Make an angled cut at the other end of the tube to ease vessel eversion.
- Submerge the cuffs in 70% ethanol followed by two washes with heparinized saline.
- Prepare a male Sprague-Dawley rat (275-350 g) for surgery.
- Seven days prior to surgery, begin administering a subcutaneous daily dose of cyclosporine (10 mg kg-1) to achieve immune suppression.
- Prior to surgery, anesthetize the animal using 3% isoflurane inhalation according to institutional SOP using an induction chamber. Verify deep anesthesia by pinching both feet and checking for reflexes.
- Administer a subcutaneous dose of analgesic buprenorphine (0.03 mg kg-1) and anticoagulative heparin (200 IU kg-1).
- Apply a lubricating eye ointment to the animal's eyes to prevent dehydration.
- Shave the surgical area of the rat and disinfect the site with iodine and then 70% ethanol. Secure the animal's limbs to the table using adhesive tape.
- Expose and isolate the common femoral artery.
- Make a 2 cm long, oblique incision through the skin, along the concavity between the leg and the abdomen.
- Using forceps and blunt-ended scissors, release the skin from the underlying tissue to visualize the inguinal fat pad.
- Using scissors and forceps, cut right through the fat pad around the upper, medial, and lower margins. Pay attention to not cut large vessels underneath the fat pad.
- Reflect the fat pad laterally, leaving the epigastric vessels intact. Place a wet gauze on the reflected fat pad to prevent drying and fix it in place. Ensure that the common femoral vessels are visible.
NOTE: The fat pad will be later used to apply soft pressure to the anastomoses. - Expose the femoral artery from the inguinal ligament proximally to the epigastric artery branching point distally by extracting it from its sheath.
CAUTION: There is usually a branch of the femoral artery running underneath it, commonly called Murphy's branch. Pay attention not to damage this difficult-to-see branch. - Divide the Murphy's branch by ligating it, and then ligate the femoral artery with two ligatures at the middle of the artery spaced 1 mm apart. Keep one end of the ligature suture long to use later to pull the vessel end through the cuff.
- Cut the artery between the two ligatures using adventitia scissors.
CAUTION: There should not be any bleeding when performing this cut. If the arterial end bleeds, clamp the artery and reapply a ligature. - Using the long end of the ligature suture, insert each vessel end into a polyimide cuff, as prepared in the previous step, such that the two cuffs' handles are pointing away from each other.
- After insertion, apply a clamp simultaneously on the cuff handle and the vessel, securing the cuff in place.
- Prepare a heparinized saline solution in a syringe equipped with a 27 G needle.
- Pull on the ligated vessel end and cut it close to the ligature. Immediately wash the vessel end thoroughly with the heparinized saline until no blood is visible in the lumen.
CAUTION: Ensure that the vessel end is washed well since any blood left inside will form a clot, which will be introduced to the bloodstream upon completing the procedure. This might lead to occlusion of the vessel and loss of perfusion to the leg. - Expand the lumen of the vessel by holding it by its sheath with one hand and dilating its lumen with the vessel dilator with the other hand.
- Place a loose 6-0 polypropylene circumferential suture around the cuff body. Evert the vessel end using two vessel dilators over the cuff body and secure it in place by tightening the circumferential suture.
NOTE: If any blood leaks through the clamped vessel during the manipulations, wash the lumen thoroughly with heparinized saline. - Rinse the engineered flap with heparinized saline, and then insert the vessel-lined cuff into the lumen of the vascular scaffold. Fix the scaffold in place by placing a circumferential 6-0 polypropylene suture around the scaffold and the cuff body. Do this step for both sides of the vascular scaffold.
- Wrap a 0.2 µm polystyrene membrane filter around the anastomoses site to isolate the engineered flap from the surrounding tissue.
- Release the distal clamp first; then, release the proximal clamp to reestablish blood flow through the vessel.
- To stop any bleeding through the anastomoses, reflect the fat pad back over the femoral vessels and apply gentle pressure using a sterile gauze for 3 min.
- Suture the fat pad back in place using 6-0 polypropylene; then, suture the skin using 5-0 PGA absorbable sutures.
- Clean the wound area with saline and apply iodine solution.
- For postoperative pain and animal management, prepare a 0.1% (v/v) of tramadol in drinking water. Moreover, administer a daily dose of heparin (200 IU kg-1) and cyclosporine (10 mg kg-1).
- Place the animal in a clean cage by itself placed under a heating lamp. Continue to monitor the animal until it regains sufficient consciousness to maintain sternal recumbency.
Representative Results
This protocol describes the fabrication of an engineered flap composed of a vascular scaffold (Figure 1Ai) and a bioprinted microvasculature (Figure 1Aii), which were assembled to achieve mesoscale and microscale vasculatures (Figure 1Aiii). Following the protocol, BVOH molds of the vascular scaffold were designed and 3D printed (Figure 1B,C). The obtained printed structures were visually inspected for small strands of BVOH, which might be found in the empty spaces of the molds (Figure 1D). These strands usually indicate incorrect material settings or that the BVOH has absorbed moisture. These strands should be removed as they might lead to incomplete filling of the mold and structural defects in the resulting vascular scaffold. Next, the molds were filled with PLLA:PLGA solution, followed by the freeze-drying process and the washing steps, as described in the protocol. The obtained PLLA:PLGA vascular scaffold was visually inspected to verify vessel wall integrity and lumen patency (Figure 1E).
A neutralized rhCollMA bioink at a concentration of 10 mg/mL was prepared and combined in a 1:1 ratio with the PEO:LAP solution. Human adipose microvascular endothelial cells labeled with Zs-Green1 and dental pulp stem cells were resuspended with the rhCollMA bioink, and the solution was loaded into a printing cartridge and onto the printer. Box shapes with a central channel with a rectilinear pattern (Figure 1D) were bioprinted inside the gelatin support bath. Following printing, the constructs were crosslinked, and the support bath was dissolved and washed. After 4 days of culture, the constructs were live-imaged to check for microvascular network self-assembly. Figure 1D shows an example of a highly developed HAMEC-ZsGreen1 microvascular network in the bioprinted construct.
Next, a fibronectin-coated vascular scaffold was inserted into the central channel of the printed construct (Figure 2A). The assembled constructs were cultured for 2 days, during which the cells contract the gel, attaching it firmly to the vascular scaffold. Then, the vascular scaffold was lined with HAMECs expressing tdTomato, according to the protocol. After 7 days of culture, the constructs were fixed and imaged. Figure 2B shows a side view of the assembled constructs where the endothelial cells in the bioprinted microvasculature are depicted in green, while the endothelial lining of the vascular scaffold is depicted in red. The image shows a green microvascular self-assembly in the bioprinted gel, while the vascular scaffold is lined with red endothelial cells. With higher magnification, sprouts originating from the red endothelial lining are seen sprouting and anastomosing with the bioprinted microvascular network (Figure 2C). Next, the constructs were stained for α-smooth muscle actin (SMA) as a marker for the dental pulp stem cells. Following the immunostaining, the constructs were imaged using a laser-scanning confocal microscope (Figure 2D).
Lastly, after 7 days of culture, the engineered flaps were microsurgically anastomosed to a rat's femoral artery, as described in the protocol. A video of a representative procedure can be seen in Supplemental Video S1. Figure 2E shows a representative image of a completed anastomoses prior to clamp removal, and Figure 2F shows a representative image of the anastomoses site after clamp removal and hemostasis. There should be no bleeding visible prior to wound closure.
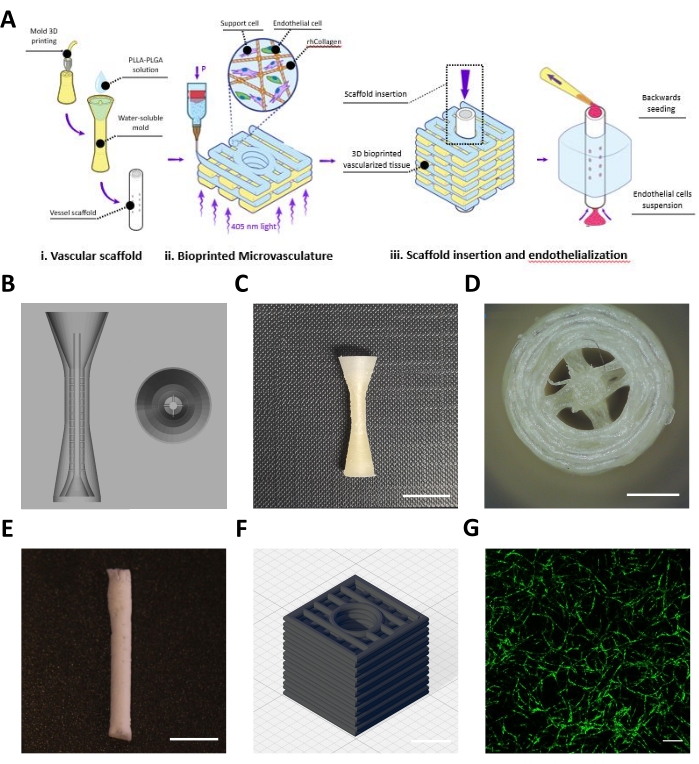
Figure 1: Representative images of the fabricated meso- and microscale vessels. (A) Schematic overview of the protocol's steps. Reproduced with permission16. (B) CAD design for the sacrificial mold of the vascular scaffold. (C) Side view of a representative 3D printed sacrificial mold (scale bar = 0.5 mm). (D) Top view of sacrificial mold (scale bar = 0.5 mm) (E) Representative vascular scaffold fabricated using the describe protocol (scale bar = 5 mm). (F) CAD design for the 3D bioprinted rhCollMA microvascular network. Grid lines = 1 mm. (G) Representative image of a highly developed bioprinted vascular network showing HAMEC-ZsGreen1 in green. Scale bar = 200 µm. Abbreviations: CAD = computer-aided design; rhCollMA = recombinant human collagen methacrylate; HAMEC = human adipose microvascular endothelial cells; PLLA = poly-L-lactic acid; PLGA = polylactic-co-glycolic acid. Please click here to view a larger version of this figure.
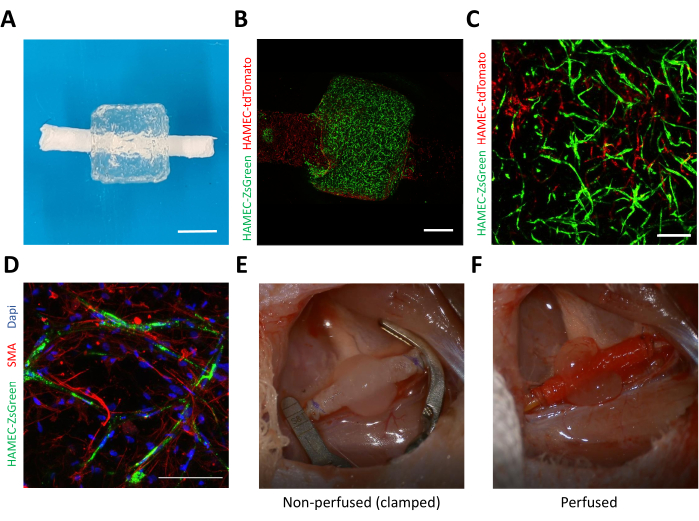
Figure 2: Representative images of the assembled vascularized flap. (A) A photograph of a representative assembly of bioprinted microvasculature and the vascular scaffold. Scale bar = 1 mm. (B) Side view of a representative engineered flap imaged 4 days after endothelial lining of the vascular scaffold. The bioprinted microvasculature is shown in green (HAMEC-ZsGreen1), while the endothelial lining is shown in red (HAMEC-tdTomato). Scale bar = 1 mm. (C) Representative image of anastomoses between the bioprinted vasculature in green and the endothelial lining in red. Scale bar = 200 µm. (D) Representative image of immunostaining for smooth muscle actin (red), nuclei (blue), and endothelial cells (green) after 7 days of incubation. Scale bar = 0.1 mm. (E) Representative image of a completed anastomoses of the engineered flap with a rat's femoral artery before clamp removal and (F) after clamp removal. Abbreviations: HAMEC = human adipose microvascular endothelial cells; SMA = smooth muscle actin; DAPI = 4',6-diamidino-2-phenylindole. Please click here to view a larger version of this figure.
Supplemental Video S1: Representative microsurgical procedure for anastomosing the vascular scaffold to a rat's femoral artery. Please click here to download this Video.
Discussion
Engineering vascularized tissues is one of the main challenges of tissue engineering20. Current methods for creating engineered vascular tissue focus on creating self-assembled microvasculature21,22,23 or fabricating mesoscale vascular scaffolds24,25 and not on recreating a system of hierarchical vasculature, which can be perfused immediately and directly upon implantation26. In this work, we describe a protocol that makes use of two 3D printing modalities to fabricate a hierarchical vessel network composed of microscale and mesoscale vasculatures. The protocol combines a 3D bioprinted, self-assembled microvascular network with a mesoscale vascular scaffold, achieving an implantable, vascularized flap. Furthermore, this paper presents a protocol for directly anastomosing this flap to a rat's femoral artery.
3D bioprinting has gained interest in recent years due to its versatility over traditional tissue engineering techniques. While this protocol describes the generation of a microvascular network in rhCollMA bioink, the methods used can be applied with few modifications to many other bioinks from the plethora of studied and novel bioinks and support baths27,28. We chose to use rhCollMA as a bioink due to the abundance of type I collagen in the human ECM, providing a suitable environment for cell attachment. Moreover, it is produced recombinantly in plants and further modified with methacrylate groups, which allows for photopolymerization and the formation of stable 3D hydrogels29,30. Photocrosslinking was achieved by the addition of the photoinitiator LAP, which has been shown to be non-toxic and is activated by exposure to 405 nm blue light, reducing the possible phototoxicity of UV light. However, the use of photosensitive bioinks necessitates the use of a phenol red-free culture medium for the preparation of the bioink and the support material. Furthermore, the protocol describes the use of gelatin support material, which enables the high-fidelity extrusion of bioinks such as rhCollMA. Thus, it is critical to ensure the use of cold medium during its preparation and the cooling of the printer bed. Excessive heating might occur due to the light source used for crosslinking or from elevated ambient temperatures.
An extrusion-based bioprinter has been used here to create the bioprinted microvascular network, and there are currently many commercially available bioprinters that can generate similar constructs. Moreover, the proposed methods may be easily modified and applied to study different geometries, sizes, and infill patterns. In this work, a rectilinear infill pattern was chosen to create interconnected pores, and this can be printed relatively quickly with high fidelity.
Air bubbles introduce a significant challenge in extrusion bioprinting, especially inside support materials. Therefore, it is crucial to minimize the presence and formation of these bubbles by using positive displacement pipettes for the transfer of the support material, the preparation of the bioink-cell suspension, and their transfer to the printing cartridges.
In this work, human adipose-derived endothelial cells and dental pulp stem cells were used as supporting cells due to their relatively easy isolation from patients. Moreover, a total cell concentration of 8 x 106 cells/mL was chosen since this concentration has been shown to establish the most developed vascular networks16. While this protocol can be employed to generate microvasculature using different cell types and sources, as well as different bioinks, a calibration of cell concentration must be done to establish the best conditions for the development of the microvascular network. Furthermore, tissue-specific cells (i.e., myoblasts or osteoblasts) can be incorporated within the bioink to achieve tissue-specific vascularized flaps.
The mold for the porous vascular scaffold was fabricated using 3D printed water-soluble material on a commercially available extrusion 3D printer. This results in a cost-effective technique based on rapid prototyping platforms, such that many different geometries and sizes of vascular scaffolds can be studied and screened rapidly31. Nevertheless, a limitation of this method is the resolution limit of most 3D printers32. However, with the rapidly evolving industry surrounding additive manufacturing, these limits are expected to improve over time. The use of organic solvents for the fabrication process is another limitation of the protocol, as most organic solvents are toxic to cells, preventing the ability to combine the bioprinting procedure with the vascular scaffold fabrication process.
The described method of seeding the lumen of the scaffold using aspiration as opposed to pushing the cell suspension has major effects on the localization of the seeded cells. Using negative pressure allows for endothelization of the inner lumen of the scaffold while minimizing any spilling of the cell suspension through the perforations on the scaffold's wall16.
The described "cuff" method for microsurgical anastomoses can be easily modified and adapted to different vascular scaffold materials or sizes, as well as to different arteries and veins in a wide scale of animal models. The adaptations to the protocol would include different polyimide tube sizes and suture sizes. This method does not require the perforation of the scaffold wall, which might lead to the development of defects. This work presents a protocol that can be expanded to many applications. The critical aspects of this protocol, which include the fabrication of meso- and microscale vasculature and their assembly and implantation, represent critical aspects of engineered flaps both for reconstructive applications, as well as vascular and other tissue engineering studies.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This project received funding from the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Programme (grant agreement no. 818808). rhCollMA was generously provided by CollPlant (Rehovot, Israel). The authors thank the Technion's Pre-clinical Research Authority for the assistance with animal care, as well as Janette Zavin, Galia Ben David, and Idan Redenski.
Materials
| 1,4-Dioxane | Biosolve Chemical | 42405 | |
| 27 G x 0.5" blunt tip dispensing needles | CML supply | 901-27-050 | |
| 3cc amber syringe barrel & piston set | Nordson EFD | 7012085 | Amber syringes used to block light and prevent premature crosslinking |
| 5-0 AssuCryl PGA absorbale suture | Assut Sutures | Absorbable sutures used for skin wound closure | |
| 6-0 polypropelene sutures | Assut Sutures | 9351 | |
| Acland clamps | S&T | B-1V | |
| Adventitia scissors | S&T | SAS-15 | |
| Angled no.3 jeweler's forceps | S&T | JFAL-3-18 | |
| BioAssemblyBot 400 3D Bioprinter | Advanced Solutions | a 6-axis 3D bioprinter | |
| Bovine albumin serum Probumin | Millipore | 82-045-1 | |
| Buprenorphine | vetmarket | B15100 | |
| BVOH filament | Verbatim | 55903 | a water-soluble 1.75 mm diameter filament |
| Clamp applying forceps | S&T | CAF-4 | |
| Dental pulp stem cells | Lonza | PT-5025 | |
| Dietrich bulldog clamps | Fine Science Tools (FST) | 18039-45 | |
| di-Sodium hydrogen phosphate (Na2HPO4) | Carlo Erba Reagents | 480141 | |
| Dissection scissors | S&T | 18039-45 | |
| DMEM, High Glucose, No Phenol Red | Sartorius | 01-053-1A | |
| Duratears | Alcon | DJ03 | |
| ECM media + bullet kit | Sciencell | #1001 | |
| Ethanol 96% | Gadot-Group | 64-17-5 | |
| GlutaMAX | Gibco | 35050061 | glutamine substitute |
| Goat anti-mouse Cy3 antibody | Jackson | 115-166-072 | |
| Heparin Sodium 5,000 I.U./mL | Panpharma | – | |
| Human adipose microvascular cells | Sciencell | #7200 | |
| Human fibronectin | Sigma | F0895-5MG | A stock concentration of 1 mg/mL |
| Isoflurane, USP Terrell | Piramal Critical Care | NDC 66794-011-25 | |
| LifeSupport | Advanced Biomatrix | 5244 | a gelatin support slurry for FRESH 3D bioprinting |
| Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) | Sigma-Aldrich | 900889 | |
| Low-glucose DMEM | Biological Industries | 01-050-1A | |
| MICROMAN E M1000E, 100-1,000 µL | Gilson | FD10006 | |
| Mouse anti-SMA antibody | Dako | M0851 | |
| NEAA | Gibco | 11140068 | |
| Needle holder | Fine Science Tools (FST) | 12500-12 | |
| Paraformaldehyde solution 4% in PBS | ChemCruz | SC-281692 | |
| Penicillin-Streptomycin-Nystatin Solution | Biological Industries | 03-032-1B | |
| Phospate buffered saline (PBS) | Sigma | P5368-10PAK | |
| Poly(ethylene oxide), M.W. 250,000 to 400,000 | Acros Organics | 178602500 | |
| Poly(L-lactic acid), IV 5.0 dl/g (PLLA) | Polysciencse, Inc. | 18582-10 | |
| Polyimide tubing, ID: 0.0249", OD: 0.0273" | Cole-Parmer | 95820-05 | A thin-walled tube used to fabricate cuffs for microsurgical anastomoses |
| Prusa I3 MK2.5 3D Printer | Prusa Research | http://www.prusa3d.com/ | a popular commercial 3D printer |
| Resomer RG 503 H, Poly(D,L-lactide-co-glycolide) (PLGA) | Evonik Industries | 719870 | |
| rhCollMA | CollPlant | https://collplant.com/ | generously provided by CollPlant (Rehovot, Israel) |
| round-handled needle holder | S&T | B-15-8 | |
| Scalpel handle – #3 | Fine Science Tools (FST) | 10003-12 | |
| small fine straight scissors | Fine Science Tools (FST) | 14090-09 | |
| Sodium Chloride | Biosolve Chemical | 19030591 | |
| Sodium Phosphate dibasic (NaH2PO4) | Riedel-de Haen | 4276 | |
| Solidworks | Dassault Systems | CAD software | |
| Straight no.3 jeweler's forceps | S&T | JF-3-18 | |
| Straight serrated forceps | Fine Science Tools (FST) | 11050-10 | |
| Surgical Scalpel Blade No.15 | Swann-Morton Limited | 305 | |
| Triton-X 100 | BioLab LTD | 57836 | |
| TSIM | Advanced Solutions | 3D slicing and design software for the BioAssembly Bot | |
| Vessel dilator | S&T | D-5a.1 | |
| Zeiss Tivato 700 surgical microscope | Zeiss |
Referencias
- Wallace, C. G., Wei, F. -. C. C. The current status, evolution and future of facial reconstruction. Chang Gung Medical Journal. 31 (5), 441-449 (2008).
- Atala, A., Kasper, F. K., Mikos, A. G. Engineering complex tissues. Science Translational Medicine. 4 (160), 12 (2012).
- Duan, B. State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Annals of Biomedical Engineering. 45 (1), 195-209 (2017).
- Kim, J. J., Hou, L., Huang, N. F. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomaterialia. 41, 17-26 (2016).
- Ouyang, L., Armstrong, J. P. K., Chen, Q., Lin, Y., Stevens, M. M. Void-free 3D bioprinting for in situ endothelialization and microfluidic perfusion. Advanced Functional Materials. 30 (1), 1908349 (2020).
- Baltazar, T., et al. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Engineering – Part A. 26 (5-6), 227-238 (2020).
- Chia, H. N., Wu, B. M. Recent advances in 3D printing of biomaterials. Journal of Biological Engineering. 9 (1), 4 (2015).
- Murphy, S. V., Atala, A. 3D bioprinting of tissues and organs. Nature Biotechnology. 32 (8), 773-785 (2014).
- Guillotin, B., et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 31 (28), 7250-7256 (2010).
- Catros, S., et al. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication. 3 (2), (2011).
- Ronca, A., Ambrosio, L., Grijpma, D. W. Preparation of designed poly(d,l-lactide)/nanosized hydroxyapatite composite structures by stereolithography. Acta Biomaterialia. 9 (4), 5989-5996 (2013).
- Lan, P. X., Lee, J. W., Seol, Y. J., Cho, D. W. Development of 3D PPF/DEF scaffolds using micro-stereolithography and surface modification. Journal of Materials Science: Materials in Medicine. 20 (1), 271-279 (2009).
- Hinton, T. J., et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science Advances. 1 (9), (2015).
- Lee, A., et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 365 (6452), 482-487 (2019).
- Bhattacharjee, T., et al. Writing in the granular gel medium. Science Advances. 1 (8), 1500655 (2015).
- Szklanny, A. A., et al. 3D bioprinting of engineered tissue flaps with hierarchical vessel networks (VesselNet) for direct host-to-implant perfusion. Advanced Materials. 33 (42), 2102661 (2021).
- Schleimer, K., et al. Training a sophisticated microsurgical technique: interposition of external jugular vein graft in the common carotid artery in rats. Journal of Visualized Experiments JoVE. (69), (2012).
- Sucher, R., et al. Mouse hind limb transplantation: A new composite tissue allotransplantation model using nonsuture supermicrosurgery. Transplantation. 90 (12), 1374-1380 (2010).
- Fensterer, T. F., Miller, C. J., Perez-Abadia, G., Maldonado, C. Novel cuff design to facilitate anastomosis of small vessels during cervical heterotopic heart transplantation in rats. Comparative Medicine. 64 (4), (2014).
- Novosel, E. C., Kleinhans, C., Kluger, P. J. Vascularization is the key challenge in tissue engineering. Advanced Drug Delivery Reviews. 63 (4-5), 300-311 (2011).
- Landau, S., et al. Tropoelastin coated PLLA-PLGA scaffolds promote vascular network formation. Biomaterials. 122, 72-82 (2017).
- Szklanny, A. A., et al. High-throughput scaffold system for studying the effect of local geometry and topology on the development and orientation of sprouting blood vessels. Advanced Functional Materials. 1901335, 1-13 (2019).
- Song, W., et al. Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nature Communications. 10 (1), 1-12 (2019).
- Luo, Y., Lode, A., Gelinsky, M. Direct plotting of three-dimensional hollow fiber scaffolds based on concentrated alginate pastes for tissue engineering. Advanced Healthcare Materials. 2 (6), 777-783 (2013).
- Ying, G. L., et al. Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Advanced Materials. 30 (50), 1-9 (2018).
- Rouwkema, J., Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends in Biotechnology. 34 (9), 733-745 (2016).
- Jang, J., Park, J. Y., Gao, G., Cho, D. W. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials. 156, 88-106 (2018).
- Suntornnond, R., An, J., Chua, C. K. Roles of support materials in 3D bioprinting – present and future. International Journal of Bioprinting. 3 (1), 83-86 (2017).
- Shoseyov, O., Posen, Y., Grynspan, F. Human recombinant type I collagen produced in plants. Tissue Engineering – Part A. 19 (13-14), 1527-1533 (2013).
- Stein, H., et al. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules. 10 (9), 2640-2645 (2009).
- Kaplan, B., et al. Rapid prototyping fabrication of precision polyester scaffolds for axonal guidance. Biomaterials. , 120062 (2020).
- Karakurt, I., Lin, L. 3D printing technologies: techniques, materials, and post-processing. Current Opinion in Chemical Engineering. 28, 134-143 (2020).

