Retrograde Parotid Gland Infusion through Stensen’s Duct in a Non-Human Primate for Vectored Gene Delivery
Summary
Salivary glands have been proposed as a tissue target site for gene therapy, especially in the area of vaccination by gene transfer. We demonstrate gene delivery in a non-human primate model utilizing retrograde parotid infusion.
Abstract
Salivary glands are an attractive tissue target for gene therapy with promising results already leading to human trials. They are inherently capable of secreting proteins into the bloodstream and are easily accessible, making them potentially superior tissue sites for replacement hormone production or vaccination by gene transfer. Suggested methods for gene delivery include transcutaneous injection and retrograde infusion through salivary ducts. We demonstrate how to perform Retrograde Salivary Gland Infusion (RSGI) in non-human primates. We describe the important anatomic landmarks including identification of the parotid papilla, an atraumatic method of cannulating and sealing Stensen's Duct utilizing basic dental tools, polyethylene tubing, and cyanoacrylate, and the appropriate rate of infusion. While this is the least traumatic method of delivery, the method is still limited by the volume able to be delivered (<0.5 mL) and the potential for trauma to the duct and gland. We demonstrate using fluoroscopy that an infusate can be fully delivered into the gland, and further demonstrate by immunohistochemistry the transduction of a typical vector and expression of the delivered gene.
Introduction
While salivary glands are well known for their exocrine production of saliva, researchers have long recognized their ability to secrete proteins directly into the bloodstream1, making them a potential target for gene therapy for systemic administration, such as replacement hormones or antibody production. In fact, salivary glands offer several advantages over other tissue targets, such as the inherent ability to produce proteins for secretion (a property muscles lack), heavy encapsulation that can limit vector diffusion, and well-differentiated tissue providing stability for non-integrating vectors. Furthermore, in the event of a serious adverse event, salivary glands are not critical for life and can be surgically removed. While not immediately intuitive, parotid glands are also easily accessible from the mouth through their main excretory duct, Stensen's Duct2.
Given the advantages of salivary tissue for gene therapy, there is increasing interest in exploring this tissue target. Numerous studies have already been performed in rodent, canine, and non-human primate models and at least one human clinical trial is underway3,4,5. To further explore and develop the utility of this tissue target for gene therapy purposes, more non-human primate studies will need to be performed. This paper describes a method for accessing the parotid glands through Stensen's Duct to deliver a vectored gene for transduction in the non-human primate model. To visibly demonstrate the delivery of the infusate and the anatomy of the duct as it enters the gland, fluoroscopy using radiocontrast was performed. To demonstrate successful transduction of a vector, an Adenovirus serotype 5 (Ad5) vectored egfp gene was used. Ad5 is a well-described vector capable of transducing salivary tissue. Although it is too immunogenic for ultimate clinical use, an Ad5 vector was chosen for this demonstration study to assure efficient transduction. Evaluating Enhanced Green Fluorescent Protein (EGFP) production is a well-described method to demonstrate successful transcription and translation of a vectored gene following transduction and was done here.
Protocol
All procedures were performed at Wake Forest School of Medicine Clarkson Campus for animal studies. The Institutional Animal Care and Use Committee (IACUC) was consulted for ethical considerations and details of the procedures was submitted for review. Wake Forest IACUC approved our study protocol and all procedures were done under IACUC approved protocol #A17-147.
1. Preparing the infusion device
- Cut size 10 Polyethylene Tube (PET10) into 25 cm lengths using a pair of scissors.
- Mark PET10 at 1 cm and 2 cm from one end using a black marker.
- Prefill 0.5 mL of Ad5-EGFP solution (109 viral particles/mL) into a 1 mL (tuberculin) syringe.
- Slide non-marked end of PET10 tube over 29-31 G needle attached to a syringe. It is generally easier to perform this task under magnification.
- Infuse the solution into the PET10 until tube is completely full (visible drop at free end).
- Use full standard PPE, including surgical scrubs, long sleeved gown, impermeable gloves, surgical mask, face shield, hair bonnet, and shoe covers.
2. Preparing the animal
NOTE: Cynomolgus macaques were used for the video demonstration. The anatomy of other non-human and human primates is very similar, and the protocol should be translatable to other species.
- Inject subcutaneously 0.05 mg/kg of atropine 15 min prior to the procedure to minimize salivary secretions and optimize distribution and retention of the infusate.
- Provide anesthesia using 5 mL syringes with intramuscular ketamine/midazolam (10-15 mg/kg of ketamine and 0.01-0.05 mg/kg of midazolam). Confirm proper anesthesia when the sedated animal becomes unconscious and is unable to react to stimuli.
3. Performing the procedure
- Use oral retractors to brace open mouth.
- Place the rubber pad of one end of the retractor on the hard palate behind the upper teeth on the side of the mouth opposite to the gland that will be infused. Place the rubber pad of the other end on the lower canine on the same side as upper retractor. Gently allow the spring action of the retractor to expand and open the mouth.
- Identify the parotid papilla, the opening of Stensen's Duct, on the posterior cheek, adjacent to the upper 2nd molar. This is best visualized using dental loops for magnification.
- Gently dilate the parotid papilla with the point of the conical dilator. It is best to place the point of the dilator into the center or opening of the papilla and then gently rotate it back and forth. The point should slowly enter the papilla and dilate it over approximately 20 – 30 s of gentle rotating.
- Insert the PET10 tubing into the dilated parotid papilla. This is best achieved by holding the marked end of the PET10 tube with tweezers approximately 0.5 cm from the distal end and gently inserting the tip of the tube into the dilated papilla.
- Gently advance the tube, which is often facilitated by small rotating movements to help the tube slide, followed by readjustment of the tweezers 0.5 cm proximal to the previous grip. Repeat this until the 2 cm mark reaches the parotid papilla.
- Apply cyanoacrylate on the cheek around the papilla and the inserted tube and wait for it to dry (no specific amount recorded, just enough to seal the entrance of Stensen's duct papilla). This typically takes less than a minute and helps seal the parotid papilla and reduce spillage of infusate back in the oral cavity.
- Slowly push the syringe content over 5 min at a rate of 100 µL/min. This slow infusion rate minimizes risk of duct injury due to sudden increase in intra-ductal pressure.
- Leave PET10 in place for at least 5 min after infusion is complete. Keep the duct sealed and allow the infusate to remain in the parotid gland.
- Remove PET10 with gentle traction. The cyanoacrylate will pull free with the tube.
- Repeat steps 3.2 through 3.8 on the opposite side.
- Slowly release oral retractors after both parotids have been infused and both PET10 tubes removed.
NOTE: The whole procedure for both sides should take less than 30 min.
4. Post-procedural care
- After infusion is completed and Stensen's duct decannulated, observe animals until anesthesia effect wears off (usually between 20-30 min post-procedure).
- Offer the animal drinks and then food after they are fully awake and resume routine care.
Representative Results
Successful procedure, transduction and transcription
Figure 1 shows the parotid papilla adjacent to the 2nd molar on the posterior superior cheek. The image also shows the correct placement of the mouth brace, one rubber end on the hard palate and the other rubber end on the ipsilateral canine. Figure 2 shows an image taken after successful cannulation of the parotid papilla at the 2 cm mark on the PET10. Figure 3 shows a fluoroscopy image at the moment of a radiocontrast infusion demonstrating branching of the solution through Stensen's Duct and into the parotid gland. This fluoroscopic image was performed for the sole intention of demonstrating the anatomy and distribution of an infusate. Fluoroscopy is not required when performing this procedure for vector delivery. Figure 4 shows EGFP immunostained in red on histopathology. Both ductal and acinar cells have been stained in red, indicating successful transduction and transcription in both cell types. In summary, these four figures demonstrate appropriate RSGI with visualization of the anatomy and of transduction of Ad5 vectored EGFP.
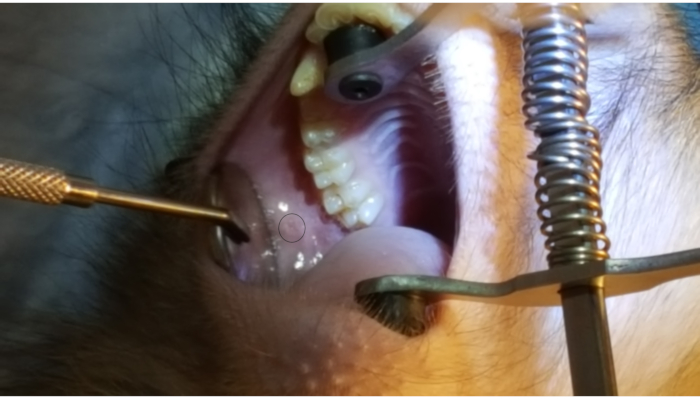
Figure 1: Parotid papilla. Note the circle on the figure highlighting the parotid papilla adjacent to the 2nd molar on the posterior cheek. Also note the placement of the mouth brace, with one rubber end on the hard palate and the other rubber band on the lower canine. Please click here to view a larger version of this figure.
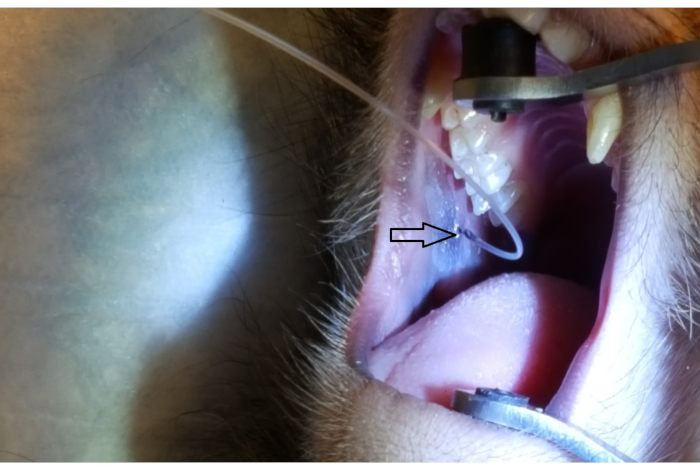
Figure 2: Parotid papilla cannulation by PET10. Note the 2 cm mark on the PET10 tube visible at the parotid papilla (arrowhead), located on the posterior cheek, adjacent to the 2nd upper molar. Please click here to view a larger version of this figure.
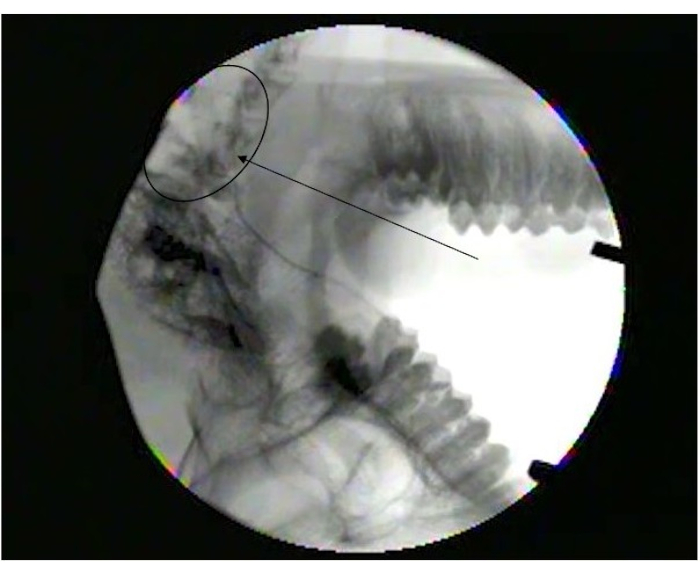
Figure 3: Fluoroscopy image showing diffusion into parotid gland. Note branching at the end of Stensen's duct (arrowhead) as it branches into smaller ducts in the parotid gland (Circle). Please click here to view a larger version of this figure.
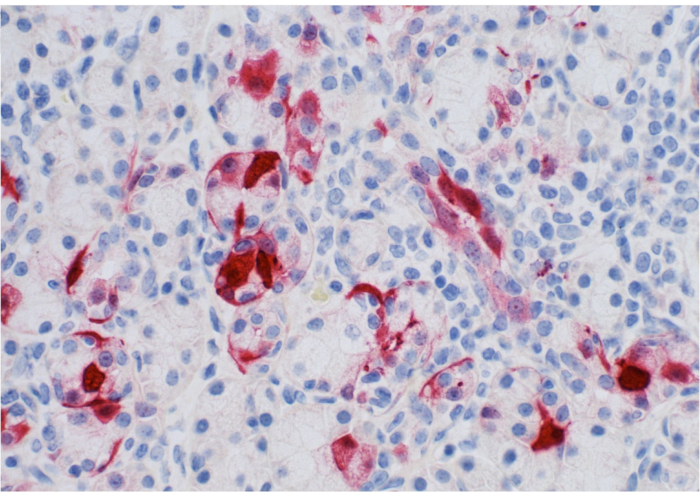
Figure 4: Pathology slide of parotid gland. Note expression of EGFP (stained in red) by ductal/acinar parotid tissue. Please click here to view a larger version of this figure.
Discussion
Here we describe a protocol of retrograde infusion into the parotid gland through Stensen's Duct. The methodology described offers guidance that can potentially be used by researchers exploring the utility of salivary tissue as a site for gene therapy and other applications.
There are multiple critical steps to ensure the success of the procedure. First and foremost, all the procedural steps should be completed gently. Forceful bracing of the mouth could result in mandibular subluxation. Forceful cannulation of the parotid papilla or rapid infusion of the solution into Stensen's Duct could result in acute ductal tears or chronic ductal stenosis.
Secondly, ensure that anesthesia has been administered and is effective. Without proper anesthesia, none of the steps can be easily accomplished and risk of animal and human injuries are significantly increased. We opted for intramuscular anesthesia with ketamine and midazolam, which is a standard regimen in non-human primate studies6. We consider atropine to be important for reducing salivary secretions during the procedure, improving visibility of the anatomy and reducing washout of the infusate prior to transduction7,8.
A step that is often challenging is the initial cannulation and advance of the PET10 into the parotid papilla and Stensen's Duct. Gentle rotation of the PET10 while inserting facilitates these steps. Excessive pushing could lead to ductal injuries.
The procedure is mainly limited by the fragility and the size of the tissue. This requires very gentle technique and use of magnifying loops and small tools to ensure proper cannulation, advance of tubing and delivery of the infusate. Another potential limitation is the volume of infusate that the parotid glands are capable of accommodating. Previous studies have infused a maximum volume of 0.5 mL into each parotid gland, totaling 1 mL per animal6,9,10. While this does not directly affect the procedure itself, depending upon the drug concentration in the infusate, it may prove limiting for a desired physiological effect.
RSGI offers the least traumatic option if salivary gland infusion is desired. Alternatives such as transcutaneous or US-guided percutaneous injections carry the risk of facial nerve injury. Furthermore, these procedures may fail to achieve adequate distribution to the entire gland, whereas RSGI utilizes the duct system to assure distribution. Fluoroscopy was performed with standard radiocontrast solution solely for the purpose of this article to demonstrate that RSGI delivers a full infusate with good distribution throughout the whole gland. This was performed separately from the actual infusion of the Ad5 vector. Fluoroscopy and/or other X-ray imaging performed during RSGI for delivering gene vectors would not be helpful and is not recommended.
As the field of therapeutics by gene transfer continues to evolve, salivary glands as a target tissue are already gaining popularity2,5. Ponzio et al. offer a great review about the advantages of the salivary glands as targets for immunization4. As encapsulated, non-vital glandular tissue which we have demonstrated is easily accessible, the parotid glands constitute an ideal gene therapy platform. RSGI offers the least traumatic technique for gene transfer into the glands.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors want to thank Mr. Cagney Gentry for his audiovisual support in filming the procedure. We also want to acknowledge the Hefner VA medical center for academic support in pursuit of this project.
Materials
| 500 µL U100 syringes with 30-gauge needles | Becton Dickinson | 328466 | fixed needle for less waste |
| Adhesive (e.g., Ethicon Dermabond) | Various | Cyanoacrylate adhesive to seal and keep the tubing in the duct during infusion. | |
| Atropine injectable solution | Patterson Veterinary | 07 869-6061 | Atropine inj. 0.54 mg/mL |
| BD Ultra-Fine Insulin Syringes 30G | Walmart | N/A | Avilable in 0.5 mL and 1.0 mL sizes. |
| Cyanoacrylate (medical glue) | Ethicon | DNX12 | Dermabond topical skin adhesive |
| Dental loops with light | Amazon (DDP) | B012M3IV80 | Used to enhance visualization of Stensen's duct papilla |
| Infant Lacrimal Dilator | Surgipro | SPOI-137 | |
| Ketamine injectable solution | Patterson Veterinary | 07-803-6637 | Ketaset inj. 100 mg/mL |
| Lacrimal Dilator | Surgipro | SPOI-132 | Used to dialate the Stensen's duct. |
| Midazolam injectable solution | Patterson Veterinary | 07 890-6698 | Midazolam inj. 5mg/mL |
| Pair of scissors | Amazon (DDP) | N/A | Used to cut PET10 tube |
| Polyethylene Tubing (PE-10) | Scientific Comodities, Inc | BB31695-PE/1 | Tubing connecting the 30G syringe and inserted into the duct. |
| Q-tips | Walmart | N/A | Used to spread cyanoacrylate on the cheek |
| Size 10 Polyethylene Tube (PET 10) | Scientific Commodities | BB31695-PE/1 | low density polyethylene tubing |
| Small Animal Mouth Opener | Amazon (DDP) | B01F3LVJXC | Used to keep the animal's mouth open. |
| Tweezers | Amazon (DDP) | N/A | Used to insert PET10 tube into Stenson's duct |
| Zinc Chloride | Sigma-Aldrich | 7646-86-7 | Included in plasmid DNA infusates |
Referencias
- Isenman, L., Liebow, C., Rothman, S. The secretion of mammalian digestive enzymes by exocrine glands. The American Journal of Physiology. 276, 223-232 (1999).
- Perez, P., et al. Salivary epithelial cells: An unassuming target site for gene therapeutics. The International Journal of Biochemistry & Cell Biology. 42, 773-777 (2010).
- Kochel, T. J., et al. A dengue virus serotype-1 DNA vaccine induces virus neutralizing antibodies and provides protection from viral challenge in Aotus monkeys. Vaccine. 18, 3166-3173 (2000).
- Ponzio, T. A., Sanders, J. W. The salivary gland as a target for enhancing immunization response. Tropical Diseases, Travel Medicine and Vaccines. 3, 4 (2017).
- Baum, B. J., et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proceedings of the National Academy of Sciences of the United States of America. 109, 19403-19407 (2012).
- Voutetakis, A., et al. Sorting of Transgenic Secretory Proteins in Rhesus Macaque Parotid Glands After Adenovirus-Mediated Gene Transfer. Human Gene Therapy. 19, 1401-1405 (2008).
- Niedzinski, E. J., et al. Enhanced systemic transgene expression after nonviral salivary gland transfection using a novel endonuclease inhibitor/DNA formulation. Gene Therapy. 10, 2133-2138 (2003).
- Niedzinski, E. J., et al. Zinc Enhancement of Nonviral Salivary Gland Transfection. Molecular Therapy. 7, 396-400 (2003).
- Samuni, Y., Baum, B. J. Gene delivery in salivary glands: From the bench to the clinic. Biochimica et Biophysica Acta – Molecular Basis of Disease. , (2011).
- Voutetakis, A., et al. Adeno-Associated Virus Serotype 2-Mediated Gene Transfer to The Parotid Glands of Nonhuman Primates. Human Gene Therapy. 18, 142-150 (2007).

