Fluorescent Leakage Assay to Investigate Membrane Destabilization by Cell-Penetrating Peptide
Summary
The fluorescence leakage assay is a simple method that enables the investigation of peptide/membrane interactions in order to understand their involvement in several biological processes and especially the ability of cell-penetrating peptides to disturb phospholipids bilayers during a direct cellular translocation process.
Abstract
Cell-penetrating peptides (CPPs) are defined as carriers that are able to cross the plasma membrane and to transfer a cargo into cells. One of the main common features required for this activity resulted from the interactions of CPPs with the plasma membrane (lipids) and more particularly with components of the extracellular matrix of the membrane itself (heparan sulphate). Indeed, independent of the direct translocation or the endocytosis-dependent internalization, lipid bilayers are involved in the internalization process both at the level of the plasma membrane and at the level of intracellular traffic (endosomal vesicles). In this article, we present a detailed protocol describing the different steps of a large unilamellar vesicles (LUVs) formulation, purification, characterization, and application in fluorescence leakage assay in order to detect possible CPP-membrane destabilization/interaction and to address their role in the internalization mechanism. LUVs with a lipid composition reflecting the plasma membrane content are generated in order to encapsulate both a fluorescent dye and a quencher. The addition of peptides in the extravesicular medium and the induction of peptide-membrane interactions on the LUVs might thus induce in a dose-dependent manner a significant increase in fluorescence revealing a leakage. Examples are provided here with the recently developed tryptophan (W)- and arginine (R)-rich Amphipathic Peptides (WRAPs), which showed a rapid and efficient siRNA delivery in various cell lines. Finally, the nature of these interactions and the affinity for lipids are discussed to understand and to improve the membrane translocation and/or the endosomal escape.
Introduction
After their discovery in the nineties, cell-penetrating peptides (CPPs) were developed to promote an efficient cellular delivery of cargoes through the plasma membrane1,2. CPPs are usually short peptides, generally 8 to 30 amino acids, having a wide variety of origins. They were first defined as "direct-translocating" carriers, meaning they were able to cross the plasma membrane and to transfer a cargo into cells independently of any endocytotic pathway neither energy requirement nor receptor involvement. However, further investigations revealed that these first observations mainly came from fluorescence overestimation due to the experimental artefact and/or to fixation protocols using methanol3. Nowadays, it is widely accepted that CPP uptake takes place by both endocytosis and energy-independent translocation4,5,6,7 depending on different parameters such as the nature of cargo, the used link between CPP and cargo, the studied cell line, etc.
CPPs can be used as transfection agents according to two strategies, either involving a chemical link (covalent strategy) or electrostatic/hydrophobic interactions (non-covalent strategy) between the CPP and its cargo8,9,10,11. Although both strategies have shown their efficiency in the cell transfer of several cargoes, the understanding of the mechanism of internalization by CPPs is still under controversy and the balance between endocytosis pathways or direct penetration is still difficult to measure12,13. Although a set of experimental tools and strategies are available to clearly address the involvement of endocytic processes, the direct translocation seems, however, more difficult to characterize since it implies more discrete interactions with plasma membrane components. Biological membranes are usually composed of numerous components, from phospholipids to membrane proteins, which might vary according to the cellular type and/or the environment (stress conditions, cell division, etc.). This diversity of composition, and consequently the absence of a universal cellular membrane model does not enable studies in a single way. However, to circumvent these limitations step-by-step approaches were developed with artificial membrane or membrane extracts. From small unilamellar vesicles to monolayer approaches, every model was clearly pertinent to answer specific questions14,15. Among them, large unilamellar vesicles (LUVs) constitute an appropriate membrane mimicking model to study peptide/membrane interactions as being a key point in the internalization process.
In this context, the following protocol describes the investigation of the effects of peptides and peptide/membrane interactions on LUVs integrity through the monitoring of both an anionic fluorescent dye and its corresponding poly-cationic quencher encapsulated in liposomes. This tool is used to study CPP/membrane interactions in order to understand whether they are able to perform a direct membrane translocation. Although usually applied to compare different membrane-interacting peptides, this LUV fluorescence leakage assay could also be used for investigating both CPPs-cargo conjugates (covalent strategy) and CPP:cargo complexes (non-covalent strategy).
The present protocol will hence be first exemplified with the recently developed tryptophan (W)- and arginine (R)-rich Amphipathic Peptides (WRAP)16. WRAP is able to form peptide-based nanoparticles to rapidly and efficiently deliver small interfering RNA (siRNA) in several cell lines16. The fluorescence leakage properties of WRAP peptide alone or siRNA-loaded WRAP-based nanoparticles were monitored to characterize their mechanism of cellular internalization. We showed that their mechanism of internalization mainly involved direct translocation7. In a second example, the WRAP peptide was covalently conjugated to the protein/protein interfering peptide iCAL36 (WRAP-iCAL36)17 and its ability to destabilize membranes was compared in a fluorescence leakage assay to iCAL36 coupled to Penetratin18 (Penetratin-iCAL36), another CPP.
Finally, the advantages and limitations of the method will be discussed both from a technological point of view and with respect to biological relevance.
Protocol
1. Preparation of Large Unilamellar Vesicles (LUVs)
- Prepare LUVs for their use as cell membrane mimics for fluorescence leakage assay.
- Mix with a Hamilton glass syringe phosphatidylcholine (DOPC, 786.11 g/mol), sphingomyelin (SM, 760.22 g/mol) and Cholesterol (Chol, 386.65 g/mol) at the molar ratio 4:4:2. The lipid solution is obtained from a stock solution of each lipid solubilized in a methanol/chloroform (3/1; volume/volume) solvent at 25 mg/mL in a 25 mL glass round-bottom flask. Based on 4 µmol of DOPC, 4 µmol of SM, and 2 µmol of Chol, the lipid solution is obtained from stock solution by mixing 126 µL, 117 µL, and 31 µL, respectively.
CAUTION: Methanol is a toxic and inflammable solvent and chloroform is toxic and carcinogenic. Both should be handled with the appropriate protection under a hood. - Evaporate methanol/chloroform using a rotary evaporator under vacuum during 45-60 min at 60 °C until formation of a dried lipid film.
- Prepare two stock HEPES buffer solutions. Prepare HEPES buffer 1 by mixing 20 mM HEPES (238.3 g/mol) and 75 mM NaCl (58.44 g/mol) and adjust pH to 7.4. Prepare HEPES buffer 2 by mixing 20 mM HEPES and 145 mM NaCl and adjust pH to 7.4. HEPES buffers can be stored at 4 °C for 1 month.
NOTE: It is recommended to check the osmolarity of the buffers using an osmometer. - Prepare lipid hydration solution by dissolving membrane impermeable fluorescent dye-quencher couple, 8-aminonaphthalene-1, 3, 6-trisulfonic acid, disodium salt at 12.5 mM (ANTS, 427.33 g/mol) and p-xylene-bispyridinium bromide at 45 mM (DPX, 422.16 g/mol) in HEPES buffer solution. Mixing ANTS with DPX leads to a yellow-colored solution. To achieve the concentrations of 12.5 mM of ANTS and 45 mM of DPX, dissolve 21.4 mg and 76 mg, respectively in 4 mL of HEPES buffer 1.
NOTE: Lipid hydration solution can be stored for 2 weeks at 4 °C by wrapping the tube with aluminum foil. - Reconstitute multilamellar vesicles (MLV) by resuspending the dried lipid film with 1 mL of the lipid hydration solution and by vortexing until dissolution of the dried lipid film. Ensure that the solution is completely solubilized as small lipid aggregates will negatively impact the preceding steps. Also, check the wall of the glass round-bottom flask to ensure that there is no remaining lipid film.
NOTE: The solution will appear opalescent and light yellow after the solubilization. - Subject the vesicles to five freeze/thaw cycles to obtain unilamellar vesicles. Perform each cycle by putting the glass round-bottom flask for 30 s in liquid nitrogen for freezing step, then leaving it in a water bath for 2 min for thawing step.
NOTE: The temperature of the bath water should be 5-10°C higher than the melting temperature of the lipids. - Prepare lipid extruder by inserting two filter supports preliminary humidified with HEPES buffer in each polytetrafluoroethylene (PTFE) extruder piece placed in the metal extruder canister.
- Put a HEPES humidified polycarbonate membrane (0.1 µm pore size, 25 mm diameter) on the top of one filter support.
- Assemble the two metal extruder canisters and screw them.
- Place the assembled extruder in the holder and introduce a 1 mL syringe in the appropriate hole at the extremity of each polytetrafluoroethylene extruder piece. Extrusion corresponds to the passage of the liquid tested from one syringe to the other through the polycarbonate membrane.
- Test the extruder with 1 mL of HEPES buffer loaded in one of the 1 mL syringe to ensure that there are no leaks or problems.
- Replace the 1 mL HEPES buffer with the MLV sample.
- Perform extrusion by passaging the MLV sample from one syringe to the other through the polycarbonate membrane at least 21 times to obtain uniform LUVs of same size.
NOTE: Extrusion should be performed at a temperature higher than the melting temperature of the lipid mixture.
2. Purification of LUVs
- Prepare a column purification to remove non-encapsulated ANTS and DPX excess.
- Introduce cross-linked dextran gel (G-50) resuspended in aqueous medium with 0.01% NaN3 (65 g/mol) in a liquid chromatography column (Luer Lock, Non-jacketed, 1.0 cm x 20 cm, bed volume 16 mL) up to 1 cm below the top of the colorless part of the column.
- Open the tap and let the liquid flow to settle the cross-linked dextran gel.
- Wash the column by eluting with 20 mL of HEPES buffer 2 and discard the output flow of the column.
- Close the tap once the dead volume of solvent above the column is minimized (<100 µL) but sufficient to avoid any drying of the cross-linked dextran gel.
- Place the freshly extruded LUVs (yellow) on the column and let them enter into the cross-linked dextran gel.
- Continuously add HEPES buffer 2 to the column to perform the LUV purification.
- Elute approximately 2 mL of HEPES buffer 2 (do not forget to regularly fill the top of the column to avoid drying the cross-linked dextran gel): the free yellow ANTS and DPX solution migrates slower than the liposomes.
- Start collecting purified LUVs in tubes (1.5 mL).
- Observe the drops of eluent from the column and when they become opalescent, they contain liposomes. Change the tube to recover the LUV-containing fraction.
- Elute until the drops are no longer opalescent (~1 mL). Afterwards, elute another 0.5 mL in a separate fraction and then stop eluting.
NOTE: Standards are now available in a wide range of molecular weights, as kits or individual molecular weights to calibrate the elution volume of the LUVs. - Wrap the tubes with the LUVs in aluminum foil to avoid bleaching of the fluorescence dye.
- Wash the column with 20 mL HEPES buffer 2.
- The LUVs can then be stored for a week at 4 °C.
NOTE: As LUV stability might depend on LUV concentration and composition, as well as on ionic strength, the size of the LUVs should be controlled using a dynamic light scattering (DLS) instrument (see section 4. Characterization of LUV Size and Homogeneity) before each test.
3. Quantifying the concentration of LUVs
- Estimate LUV concentration by a phospholipid quantification kit, which enables the evaluation of choline concentration19. This assay might be applied when phospholipids with choline containing polar head is substantial (>50% of the LUVs).
- Prepare the color reagent by dissolving 18 mg of chromogen substrate in 3 mL of buffer provided.
- Load a polystyrene cuvette, 10 x 10 x 45 mm, with 3 mL of color reagent.
- Use the pure color reagent as blank condition (Blank). Add 20 µL of LUV sample (Test) or 20 µL of standard solution of known choline concentration (Standard).
- Mix well and incubate for 5 min at 37 °C all conditions (Blank, Test, and Standard).
- Measure the absorbance (optical density, OD) of the test sample and standard solution with the blank solution as the control at 600 nm with a spectrophotometer.
- Check the OD values which enable to estimate the lipid concentration of the LUVs, C[LUV], in choline equivalent compared to the standard of known concentration.
- Perform the calculation using the following equation:
C[LUV] (mol / l) = (OD Sample / OD Standard) x C[Standard] (mol / l)
NOTE: The phospholipid quantification kit provided a Choline Chloride (139.6 mg/l) standard solution at 54 mg/dL corresponding to molar concentration of C[Standard] = 3.87 mmol/L. OD Sample and OD Standard are the absorbances measured at 600 nm for the LUV and Choline solutions, respectively.
4. Characterization of LUV size and homogeneity
- Perform a measurement using a DLS instrument in order to determine the LUV size (in nm) and polydispersity index (PdI).
- Program the appropriated "standard operation procedure" (SOP) by indicating the viscosity of the solvent/buffer and the used cuvette.
- Place 500 µL of the LUV solution in a polystyrene semi-micro cuvette.
- Insert the polystyrene semi-micro cuvette in a DLS instrument.
- At room temperature, measure the size distribution in terms of mean size (Z-average) of the particle distribution and of homogeneity (polydispersity index, PdI).
- All the results are obtained from two independent measurements performed each in three repetitive cycles.
NOTE: Standard values for LUVs will be a mean size of 137 ± 7 nm with a PdI of 0.149 ± 0.041.
5. Preparing peptide solutions
- Prepare a stock solution of the peptide, which should be analyzed for the leakage assay.
- Dissolve peptide powder (>95% purity) in pure water (e.g., 1 mg peptide in 500 µL pure water).
NOTE: It is recommended to dilute peptides in pure water and to avoid dimethyl sulfoxide (DMSO) solubilization, which could induce artifacts (e.g., membrane permeabilization20). - Vortex the peptide solution for 5 s.
- Sonicate the peptide solution in a water sonication bath for 5 min and then centrifuge for 5 min at 12,225 x g. Collect the supernatant for concentration determination.
- Measure the absorbance at 280 nm of three independent peptide dilutions and then calculate peptide concentration using its molar extinction coefficient ε (depending on tryptophan and tyrosine content in the peptide sequence) and Beer-Lambert rule.
NOTE: If the peptide contains tryptophan and tyrosine, the molar extinction coefficient ε is computed on the basis of Tryptophan ε = 5,690 M-1cm-1 and Tyrosine ε = 1,280 M-1cm-1. If the peptide sequence contains no tryptophan or tyrosine, other colorimetric assay could be performed to measure the concentration (e.g., BCA or Bradford). - Dilute the peptide solution in pure water to a final solution of 100 µM and store at 4 °C.
NOTE: In pure water, no peptide degradation occurs during the 4 °C storage. However, peptide concentration should be measured every 2 weeks to ensure that no water evaporation occurs.
6. Fluorescence leakage assay
- Fluorescence leakage assay is measured on a spectrofluorometer at room temperature. Excitation and emission wavelength are fixed at Ex = 360 nm ± 3 nm and Em = 530 nm ± 5 nm, respectively.
- Dilute LUVs in 1 mL HEPES buffer 2 to a final concentration of 100 µM in a quartz fluorescence cuvette. Add a magnetic stirrer to homogenize the solution during experiment.
- Measure the LUVs alone during the first 100 s, between t = 0 s and t = 99 s in order to access the background fluorescence.
NOTE: LUVs alone could also be measured during the whole experiment (15 min) in order to access background fluorescence and potential leaks. - Thereafter, measure leakage as an increase in fluorescence intensity upon addition of aliquots of peptide solution for the next 900 s (15 min). This protocol is carried out for each concentration of peptide tested from 0.1 µM to 2.5 µM.
- Finally, 100% fluorescence was achieved by solubilizing the LUVs by addition of 1 µL of Triton X-100 (0.1%, v/v), resulting in the completely unquenched probe between t = 1,000 s and t = 1,100 s.
7. Quantification of the leakage
- Suppress values obtained after t = 1,090 s in order to keep the same number of points for each tested condition.
- Calculate the minimal fluorescence, Fmin, by making the average of 50 points between t = 0 s and t = 49 s (LUVs alone).
- Calculate the maximal fluorescence, Fmax, by making the average of 50 points between t = 1,041 s and t = 1,090 s (LUVs with Triton X-100).
- Calculate the leakage percentage (%Leak) at each time point (t = x), according to the following equation:
%Leak(t=x) = (F(t=x) – Fmin) / (Fmax – Fmin) x 100 - Calculate the average and standard deviation for values obtained with different LUV preparation (n ≥ 2) for the same condition.
- Plot the leakage percentage, %Leak(t=x), in function of time (s).
Representative Results
The principle of the fluorescence leakage assay is shown in the Figure 1. In detail, large unilamellar vesicles (LUVs) encapsulating a fluorescent dye and a quencher (no fluorescence signal) are treated with the biomolecule of interest. Due to the interaction of the peptide with lipid membranes, which could imply membrane permeability, reorganization or even rupture, the fluorescence dye and the quencher are released from the LUVs. Subsequent dilutions in the buffer results in an increased fluorescence signal.
Although this scheme displays a test with free peptides, the advantage of the system lies in the ability to also test cargo-conjugated peptides, peptide-based nanoparticles or other biopolymers, which are suspected to destabilize lipid membranes. Although a preliminary optimization of the protocol especially with regards to the molecules tested is required, this fluorescence leakage assay might be extended to a huge variety of membrane-interacting components. In the present protocol, we show some results obtained with the CPPs and their complexed (non-covalent strategy) or conjugated (covalent strategy) forms. The following examples imply WRAP alone as well as siRNA-loaded WRAP-based nanoparticles and two different peptide-conjugates (WRAP-iCAL3617 and Penetratin-iCAL36).
With regard to non-covalent strategy, fluorescence leakage assay with peptides and with their corresponding siRNA-loaded nanoparticles in the presence of LUVs are displayed in Figure 2. The vesicles are composed of a mixture of DOPC/SM/Chol (4:4:2) reflecting the plasma membrane as described in Konate et al.21 and are usually used to directly evaluate the possibility of lipid bilayer interaction and/or transduction properties of free WRAP and WRAP-based nanoparticles7. In the absence of peptides, no leakage is observed (baseline during the first 100 s). Addition of free WRAP on the LUVs induces a significant increase of fluorescence revealing an important LUV leakage and ANTS release. After 15 min, a leakage of 67.8% ± 0.4% compared to the Triton condition (positive control) is obtained at the used concentration (2.5 µM) of WRAP peptide. It should be noted here that several different concentrations have also been tested and revealed a dose-dependent fluorescence increase, corresponding to a dose-dependent LUV leakage7. In contrast, when WRAP is assembled at the same concentration with siRNA to form peptide-based nanoparticles, the leakage is 1.5-fold weaker (40.5% ± 0.5%) compared to the free peptide (Figure 2). Similar leakage values have been reported for the RICK peptide (60%) or the RICK:siRNA nanoparticles (28%)22. The difference in values between free peptide compared to nanoparticles might be explained by the fact that, when engaged in the nanoparticles, a substantial part of the peptide is involved in direct interactions with the siRNA, reducing the peptide availability for interactions with lipids.
Concerning the covalent strategy, fluorescence leakage assays with CPP-conjugates in the presence of LUVs are shown in Figure 3. With the same LUV composition [DOPC/SM/Chol (4:4:2)], two conjugated peptides are applied: WRAP-iCAL36 and Penetratin-iCAL36. As previously noticed, no leakage is observed in the absence of peptides. 15 min after injection of 2.5 µM of Penetratin-iCAL36, no significant fluorescence increase is detected (2.3% ± 0.7%), whereas an addition of 2.5 µM of WRAP-iCAL36 induces a net leakage characterized by very strong fluorescence signal (85.8% ± 11.1%)17. These observations indicate that for some peptides, or conjugates, no fluorescence leakage might occur, suggesting no peptide/membrane interactions or no lipid bilayer disturbance. This is in accordance with previously published results showing that Penetratin as well as Tat were not able to destabilize membranes23,24,25. It should be highlighted that the membrane destabilization properties of a CPP could change depending on the coupled cargo26.
Furthermore, although WRAP-iCAL36 caused a strong leakage, additional studies do not reveal specific cellular internalization, indicating that these conjugates remained inside the lipids bilayer of the plasma membrane17. In contrast to the WRAP nanoparticle, we suppose that the WRAP-cargo conjugate is able to destabilize the LUV membrane by sticking between the lipid chains or by forming pores.
These results indicate that the fluorescence leakage assay might reveal the ability of some CPPs to develop peptide/membrane interactions, which could lead to a more or less pronounced membrane permeability. Moreover, these interactions might occur whatever the strategy of cargoes delivery (nanoparticles versus conjugates). Conversely, this method does not discriminate whether a CPP, which does not induce any fluorescence leakage, might still interact with the bilayer or biological membrane of the lipids. This kind of behavior requires additional approaches such as zeta-potential measurements, FRET between peptide and membrane, or tryptophan fluorescence experiments to mention only a few examples.
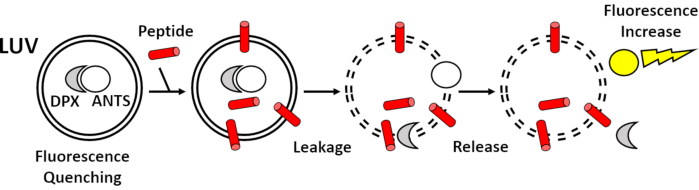
Figure 1: Principle of the fluorescence leakage assay. LUVs were loaded with a fluorescent dye (ANTS) in white and its corresponding quencher (DPX) in grey. In the absence of peptides (in red), no fluorescence signal was observed because ANTS fluorescence was quenched by DPX. Addition of peptides on the LUVs induced membrane permeability and the subsequent release of both ANTS and DPX resulting in a significant increase in ANTS fluorescence (yellow). Please click here to view a larger version of this figure.
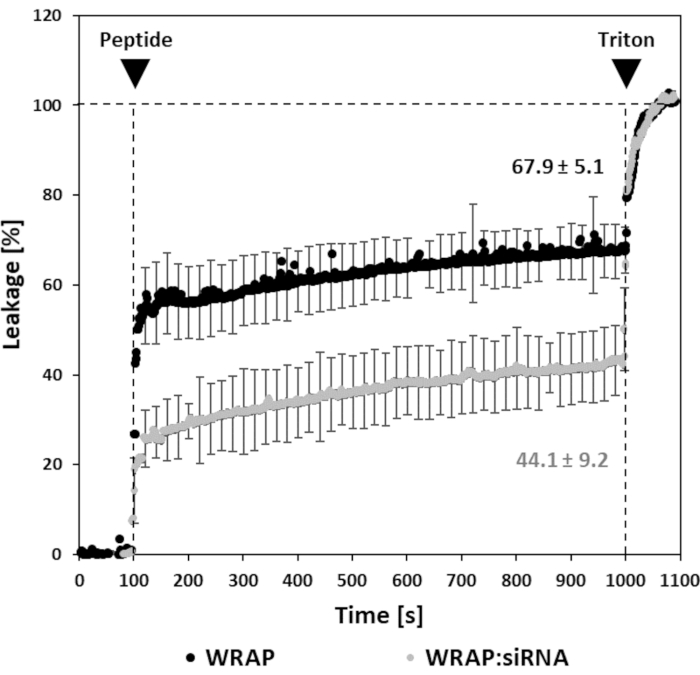
Figure 2: Fluorescence leakage assay with free WRAP and WRAP:siRNA nanoparticles. Peptide alone and siRNA-loaded nanoparticles were applied on LUVs at the peptide concentration of 2.5 µM. WRAP-based nanoparticles were formulated at a peptide:siRNA molar ratio of 20 (R 20) as described in Konate et al.7,16. Black arrows show injections of peptide (nanoparticle) and Triton X-100, respectively. Please click here to view a larger version of this figure.
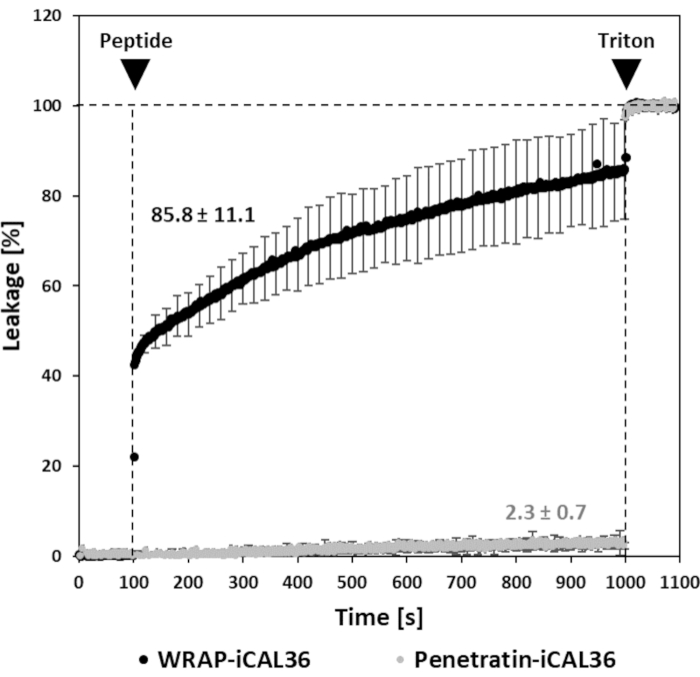
Figure 3: Fluorescence leakage assay with WRAP-iCAL36 and Penetratin-iCAL36 conjugates. Conjugates were applied on LUVs at the concentration of 2.5 µM. Black arrows show injections of peptide and Triton X-100, respectively. Please click here to view a larger version of this figure.
Discussion
The presented fluorescence leakage assay is a simple and fast method to address membrane destabilization by cell-penetrating peptide. Easy to do, it also enables an indirect comparison between different membrane-interacting peptides or other membrane-interacting molecules. Concerning critical steps of the protocol, as this assay provides relative values between the baseline (LUVs alone) and maximal fluorescence release (Triton condition), we usually evaluate the concentration of LUVs using the phospholipid quantification kit, which only estimates choline contribution of the LUVs. However, it is also possible to include a more accurate measurement of LUV concentration by determining the total phosphorus content by acid digestion as described by Rouser and colleagues27 or by Bartlett28 or by colorimetric method using ammonium ferrothiocyanate complexing phospholipids29. In our hands, it does not have an impact on the relative quantification of leakage and on the comparison between membrane-interacting peptides.
With regard to the use of LUVs, one should also notice the importance to control the state of the baseline of LUV alone in order to check whether they are usable and if they are already showing leaks (characterized by a continuous increase of fluorescence). In the same way, it is important to measure the maximal fluorescence release after each peptide incubation to ensure that no false-negative results were recorded due to an undesired quenching process (e.g., peptide/dye quenching).
In general, according to DLS characterization, LUVs are quite stable for 1 week and should not display a too high fluorescence background. In this context, purification of ANTS/DPX loaded liposomes by gel filtration is also a key point since enabling formation of LUVs without having residual fluorescence that might contribute to the background or produce fluorescence overestimation. Additionally, it is strongly recommended to calibrate the protocol by including a specific positive control that can always give the same leakage percentage. This constitutes an internal control through the different measurements, which might reinforce characterization of the LUVs for each test and increase statistics.
Although the fluorescence leakage assay might provide a fast comparison in membrane-destabilization by CPPs, it is however limited in the interpretation of peptide-membrane interactions since some peptides are able to interact with lipid bilayer without inducing any membrane disturbance or leakage. For these peptides, it is highly recommended to use additional experiments with specific fluorescence markers enabling FRET30.
It should be also noted that other fluorescence dye/quencher pairs (e.g., Tb3+/DPA31) could be used. Both the methods have their inconveniences: Terbium (III) is not very soluble in water and cannot be used in the presence of phosphate, whereas the ANTS quenching is not a linear function of DPX concentration. Furthermore, other self-quenching materials such as 70 mM Calcein solution32 or dextran-PTS33 could be handled depending on the membrane defects provoked by the analyzed peptide or compound.
Finally, the main advantage of this fluorescence leakage assay is the ability to test a multitude of potential membrane interacting molecules as well as different membrane compositions, such as endosomal membrane mimics [e.g., dioleoylphosphatidylcholine (DOPC)/dioleoyl-phospha-tidylethanolamine (DOPE)/phosphatidylinositol from soybean (PI)/bis(monooleoylglycero) phosphate (LBPA) at a molar ratio of 5:2:1:2]34, mitochondrial membrane mimics [e.g., 46.5% DOPC, 28.5% phosphatidylethanolamine (PE), 9% phosphatidylinositol (PI), 9% phosphatidylserine (PS), and 7% cardiolipin (CL)35 or any other desired lipid bilayer composition. However, one will first have to ensure stability of the vesicles (no LUV fusion, aggregation, or precipitation, no lipidic falling out of suspension, no negative membrane curvature, etc.) having a constant mean size close to 100 nm during experiment and storage.
In addition, the advantage of this method compared to extracted membranes (red blood cells, mitochondria, etc.) is the use of purified well-characterized lipids in the absence of proteins. The control of the lipid bilayer composition (plasma, endosomal, or mitochondrial membrane) also enables the insertion of specific membrane proteins (proton pump). Moreover, the ability to control both LUVs internal and external environments allows a clear interpretation of membrane disturbance/leakage. Compared to black-lipid membrane experiments36, which can also visualize membrane permeabilization events, this leakage protocol provides a more simple screening method of membrane-active peptides.
In conclusion, this simple method favors a rapid identification of strong peptide/membrane interactions leading to membrane destabilization. It may be applied to investigate the mechanism of internalization of CPPs and in a more general approach of "membrane-active peptides" such as fusiogenic or antimicrobial peptides.
In general, the characterization of the main route used by CPPs to reach the cytoplasm is very complex and requires several distinct approaches, from biophysics to cellular biology. For example, the investigation of WRAP internalization revealed a balance between different mechanisms to enter the cells (endocytosis versus direct translocation) and the fluorescence leakage assay, associated to other methods, has contributed to support a direct penetration process7.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Emilie Josse for the critical review of the manuscript. This work was supported by the foundation "La Ligue contre le Cancer", the "Fondation ARC pour la Recherche sur le Cancer", and the "Centre National de la Recherche Scientifique" (CNRS).
Materials
| 25 mL glass round-bottom flask | Pyrex | ||
| 8-aminonaphthalene-1, 3, 6-trisulfonic acid, disodium salt (ANTS) | Invitrogen | A350 | Protect from light |
| Chloroform | Sigma-Aldrich | 288306 | |
| Cholesterol | Sigma-Aldrich | C8667 | |
| DOPC (dioleoylphosphatidylcholine) | Avanti Polar | 850375P | Protect from air |
| Extruder | Avanti Polar | 610000 | |
| Fluorimeter | PTI Serlabo | ||
| 50 µL glass syringe | Hamilton | 705N | |
| HEPES | Sigma-Aldrich | H3375 | |
| LabAssay Phospholipid | WAKO | 296-63801 | |
| liquid chromatography column | Sigma-Aldrich | ||
| Methanol | Carlo Erba | 414902 | |
| Nuclepore polycarbonate membrane (0.1 µm pore size, 25 mm diameter) | Whatman | 800309 | |
| polystyrene cuvette, 10 x 10 x 45 mm | Grener Bio-One | 614101 | |
| polystyrene semi-micro cuvette, DLS | Fisher Scientific | FB55924 | |
| p-xylene-bispyridinium bromide (DPX) | Invitrogen | X1525 | Protect from light |
| quartz fluorescence cuvette | Hellma | 109.004F-QS | |
| rotavapor system | Heidolph | Z334898 | |
| Sephadex G-50 resin | Amersham | 17-0042-01 | |
| Sodium azide (NaN3) | Sigma-Aldrich | S2002 | |
| Sodium chlorid (NaCl) | Sigma-Aldrich | S5886 | |
| Sonicator bath USC300T | VWR | 142-6001 | |
| Sphingomyelin | Avanti Polar | 860062P | Protect from air |
| Triton X-100 | Eromedex | 2000-B | |
| Zetaziser NanoZS | Malvern | ZEN3500 |
Referenzen
- Langel, U. . Handbook of Cell-Penetrating Peptides. , (2006).
- Deshayes, S., Morris, M. C., Divita, G., Heitz, F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cellular and Molecular Life Sciences CMLS. 62 (16), 1839-1849 (2005).
- Richard, J., et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. The Journal of Biological Chemistry. , (2003).
- Jones, A., Sayers, E. Cell entry of cell penetrating peptides: tales of tails wagging dogs. Journal of Controlled Release Official Journal of the Controlled Release Society. , (2012).
- Tünnemann, G., et al. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. Journal of Peptide Science. 14 (4), 469-476 (2008).
- Jiao, C. -. Y., et al. Translocation and endocytosis for cell-penetrating peptide internalization. Journal of Biological Chemistry. 284 (49), 33957-33965 (2009).
- Deshayes, S., et al. Deciphering the internalization mechanism of WRAP:siRNA nanoparticles. Biochimica Et Biophysica Acta. Biomembranes. 1862 (6), 183252 (2020).
- Konate, K., et al. Optimisation of vectorisation property: A comparative study for a secondary amphipathic peptide. International Journal of Pharmaceutics. 509 (1-2), 71-84 (2016).
- Lehto, T., et al. Cellular trafficking determines the exon skipping activity of Pip6a-PMO in mdx skeletal and cardiac muscle cells. Nucleic Acids Research. 42 (5), 3207-3217 (2014).
- Hoyer, J., Neundorf, I. Peptide vectors for the nonviral delivery of nucleic acids. Accounts of Chemical Research. 45 (7), 1048-1056 (2012).
- Milletti, F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discovery Today. 17 (15-16), 850-860 (2012).
- Mueller, J., Kretzschmar, I., Volkmer, R., Boisguerin, P. Comparison of cellular uptake using 22 CPPs in 4 different cell lines. Bioconjugate Chemistry. 19 (12), 2363-2374 (2008).
- Ramaker, K., Henkel, M., Krause, T., Röckendorf, N., Frey, A. Cell penetrating peptides: a comparative transport analysis for 474 sequence motifs. Drug Delivery. 25 (1), 928-937 (2018).
- Maget-Dana, R. The monolayer technique: a potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochimica Et Biophysica Acta. 1462 (1-2), 109-140 (1999).
- Alves, A. C., Ribeiro, D., Nunes, C., Reis, S. Biophysics in cancer: The relevance of drug-membrane interaction studies. Biochimica et Biophysica Acta (BBA) – Biomembranes. 1858 (9), 2231-2244 (2016).
- Konate, K., et al. Peptide-based nanoparticles to rapidly and efficiently “Wrap ‘n Roll” siRNA into cells. Bioconjugate Chemistry. 30 (3), 592-603 (2019).
- Seisel, Q., Pelletier, F., Deshayes, S., Boisguerin, P. How to evaluate the cellular uptake of CPPs with fluorescence techniques: Dissecting methodological pitfalls associated to tryptophan-rich peptides. Biochimica Et Biophysica Acta. Biomembranes. 1861 (9), 1533-1545 (2019).
- Derossi, D., Joliot, A. H., Chassaing, G., Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. Journal of Biological Chemistry. 269 (14), 10444-10450 (1994).
- Takayama, M., Itoh, S., Nagasaki, T., Tanimizu, I. A new enzymatic method for determination of serum choline-containing phospholipids. Clinica Chimica Acta; International Journal of Clinical Chemistry. 79 (1), 93-98 (1977).
- Notman, R., Noro, M., O’Malley, B., Anwar, J. Molecular basis for Dimethylsulfoxide (DMSO) action on lipid membranes. Journal of the American Chemical Society. 128 (43), 13982-13983 (2006).
- Konate, K., et al. Insight into the cellular uptake mechanism of a secondary amphipathic cell-penetrating peptide for siRNA delivery. Biochemie. 49 (16), 3393-3402 (2010).
- Vaissière, A., et al. A retro-inverso cell-penetrating peptide for siRNA delivery. Journal of Nanobiotechnology. 15 (1), 34 (2017).
- Eiríksdóttir, E., Konate, K., Langel, &. #. 2. 2. 0. ;., Divita, G., Deshayes, S. Secondary structure of cell-penetrating peptides controls membrane interaction and insertion. Biochimica et Biophysica Acta (BBA) – Biomembranes. 1798 (6), 1119-1128 (2010).
- Ziegler, A., Li Blatter, X., Seelig, A., Seelig, J. Protein transduction domains of HIV-1 and SIV TAT interact with charged lipid vesicles. Binding mechanism and thermodynamic analysis. Biochemie. 42 (30), 9185-9194 (2003).
- Thorén, P. E. G., Persson, D., Karlsson, M., Nordén, B. The Antennapedia peptide penetratin translocates across lipid bilayers – the first direct observation. FEBS Letters. 482 (3), 265-268 (2000).
- Mishra, A., et al. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proceedings of the National Academy of Sciences. 108 (41), 16883-16888 (2011).
- Rouser, G., Fleischer, S., Yamamoto, A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 5 (5), 494-496 (1970).
- Bartlett, G. R. Phosphorus assay in column chromatography. Journal of Biological Chemistry. 234 (3), 466-468 (1959).
- Stewart, J. C. M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Analytical Biochemistry. 104 (1), 10-14 (1980).
- Spinella, S. A., Nelson, R. B., Elmore, D. E. Measuring peptide translocation into large unilamellar vesicles. Journal of Visualized Experiments: JoVE. (59), e3571 (2012).
- Wimley, W. C. Determining the effects of membrane-interacting peptides on membrane integrity. Cell-Penetrating Peptides. 1324, 89-106 (2015).
- Bárány-Wallje, E., Gaur, J., Lundberg, P., Langel, U., Gräslund, A. Differential membrane perturbation caused by the cell penetrating peptide Tp10 depending on attached cargo. FEBS Letters. 581 (13), 2389-2393 (2007).
- van Rooijen, B. D., Claessens, M. M. A. E., Subramaniam, V. Membrane permeabilization by oligomeric α-Synuclein: in search of the mechanism. PloS One. 5 (12), 14292 (2010).
- Hassane, F. S., et al. Insights into the cellular trafficking of splice redirecting oligonucleotides complexed with chemically modified cell-penetrating peptides. Journal of Controlled Release: Official Journal of the Controlled Release Society. 153 (2), 163-172 (2011).
- Asciolla, J. J., Renault, T. T., Chipuk, J. E. Examining BCL-2 family function with large unilamellar vesicles. Journal of Visualized Experiments: JoVE. (68), e4291 (2012).
- Grewer, C., Gameiro, A., Mager, T., Fendler, K. Electrophysiological characterization of membrane transport proteins. Annual Review of Biophysics. 42 (1), 95-120 (2013).

