Self-standing Electrochemical Set-up to Enrich Anode-respiring Bacteria On-site
Summary
On-site microbial enrichment or in situ cultivation techniques can facilitate the isolation of difficult-to-culture microbial taxa, especially from low-biomass or geochemically extreme environments. Here, we describe an electrochemical set-up without using an external power source to enrich microbial strains that are capable of extracellular electron transport (EET).
Abstract
Anaerobic respiration coupled with electron transport to insoluble minerals (referred to as extracellular electron transport [EET]) is thought to be critical for microbial energy production and persistence in many subsurface environments, especially those lacking soluble terminal electron acceptors. While EET-capable microbes have been successfully isolated from various environments, the diversity of bacteria capable of EET is still poorly understood, especially in difficult-to-sample, low energy or extreme environments, such as many subsurface ecosystems. Here, we describe an on-site electrochemical system to enrich EET-capable bacteria using an anode as a respiratory terminal electron acceptor. This anode is connected to a cathode capable of catalyzing abiotic oxygen reduction. Comparing this approach with electrocultivation methods that use a potentiostat for poising the electrode potential, the two-electrode system does not require an external power source. We present an example of our on-site enrichment utilized in an alkaline pond at the Cedars, a terrestrial serpentinization site in Northern California. Prior attempts to cultivate mineral reducing bacteria were unsuccessful, which is likely due to the low-biomass nature of this site and/or the low relative abundance of metal reducing microbes. Prior to implementing our two-electrode enrichment, we measured the vertical profile of dissolved oxygen concentration. This allowed us to place the carbon felt anode and platinum-electroplated carbon felt cathode at depths that would support anaerobic and aerobic processes, respectively. Following on-site incubation, we further enriched the anodic electrode in the laboratory and confirmed a distinct microbial community compared to the surface-attached or biofilm communities normally observed at the Cedars. This enrichment subsequently led to the isolation of the first electrogenic microbe from the Cedars. This method of on-site microbial enrichment has the potential to greatly enhance the isolation of EET-capable bacteria from low biomass or difficult to sample habitats.
Introduction
Several mineral-reducing microbes have been shown to utilize solid-phase minerals as terminal electron acceptors, by extracellular electron transport (EET) processes that conduct electrons to the exterior of the cell via redox enzymes1. EET is critical, not only for microbe-mineral processes but also applied energy and environmental technologies, such as microbial fuel cells2, electrode synthesis3, and bioremediation4. New EET-capable bacteria are highly sought after, and have been extensively studied from a fundamental or applied perspective5. However, we only have limited insight into the ecological or biogeochemical significance of these bacteria. The majority of EET-capable microbes have been isolated following enrichment from aqua, sediment, or anaerobic digesters using solid electron acceptors such as MnO2, Fe2O3 or poised electrodes in laboratory6,7,8. However, these methods often produce similar consortia and potentially miss more sensitive taxa that may dominate low energy or low biomass systems, biasing the ability of these microbes to adapt to the lab or axenic culture environment9. Usually for low biomass environments, large quantities of water from a site are filtered to concentrate bacterial cells. However, EET-capable bacteria often exhibit anaerobic metabolisms and therefore oxygen exposure may further inhibit or prevent their cultivation. Alternative on-site methodologies to concentrate cells without exposing them to oxygen could facilitate the isolation of EET-capable bacteria. Here, we report setup details for an on-site electrochemical technique to enrich EET-capable microbe over a long period of time without the need for an external power source.
Using our electrocultivation experiments from a highly alkaline spring in Northern California, the Cedars10, we describe this on-site electrochemical technique. The geochemistry of the springs at The Cedars are impacted by serpentinization in the subsurface. The springs are highly reductive, with oxygen concentrations below the limit of detection under the air water interface highlighting the potential for microbial energy production via EET in this functionally anoxic environment11. However, there is no evidence to support EET-capable microbes from the Cedars (in either 16S rRNA or Metagenomic analysis). Even though this environment has been characterized as electron acceptor limited, the potential for using insoluble minerals as terminal electron acceptors, including minerals such as the iron baring minerals that result from serpentinization (i.e., magnetite), has not been extensively investigated12. We, therefore, deployed our electrochemical system at Campsite Spring, a high pH spring at the Cedars, to enrich for EET-capable microbes (Figure1)13.
Protocol
1. Construction of a Two-electrode System for Environmental Incubation
- Preparation of the anode material and treatment of Carbon felt electrode (Figure 2).
- Cut the carbon felt to equal dimensions depending on desired biomass enrichment. Soak each electrode in 90% ethanol for 30 min, then rinse at least 8 times with deionized water, sonicating for 1 min after each rinse.
- Wash the electrodes twice in 1 M HCl, stirring for a minimum of 12 h for each wash.
- Dry the electrodes in a warm (37 °C) oven for 6–12 h or until free of liquid.
- Attach electrodes to titanium wire using graphite epoxy per manufacturer's protocol on a polytetrafluoroethylene plate (non-stick surface).
NOTE: We used a titanium wire because of its high tolerance to aerobic corrosion. - Bake the electrode at 120 °C for 6 h.
- Test the resistance between titanium wire and carbon felt with an ohmmeter and confirm that the resistance between the wire and felt electrodes is less than 5 ohms.
- Electroplation of platinum on the carbon felt electrode for preparation of the cathode material
- Submerge carbon felt electrodes, prepared in step 1.1, in 2 M KOH for a minimum of 12 h in a glass container.
- For electrochemical cleaning, place the electrode as working electrode (WE) in a three-electrode reactor, which also accommodates a reference (RE) and counter electrode (CE). Connect WE, RE, and CE to the potentiostat by alligator clips. Confirm all the connections with an ohmmeter.
NOTE: We used an Ag/AgCl (KCl saturated) electrode and a platinum wire as RE and CE, respectively. - Poise the electrode at 1.0 V vs. Ag/AgCl for 600 s in electrolyte solution containing 2 M KOH (using a sufficient amount to submerge the whole electrode). Take out the electrode from the electrochemical reactor (which is made of glass). Rinse the electrode in deionized water at least 8 times, sonicating for 1 min after each rinse. Dry electrodes at 100 °C for at least 12 h.
- To prepare plating solution, add 100 g of citric acid, 5 g of sodium sulfate, and 2 g of dihydrogen hexachloroplatinate (IV) hexahydrate to 1 L of 2 M sulfuric acid.
- Weigh cleaned and dried electrodes as prepared in steps 1.2.1–1.2.2, and then cover the electrode in a plating solution as prepared in step 1.2.3. Sonicate the electrode in the plating solution three times for 30 s each.
- Electroplate the electrodes by poising the electrode potential at -0.2 V vs. Ag/AgCl for 460 s in plating solution. Rinse electrodes twice in deionized water and discard the platinum waste.
- Rinse electrodes in deionized water at least 3 times, sonicating for 20 s after each rinse. Rinse without sonication at least three more times.
- Dry electrodes at 100 °C for at least 12 h. Weigh electrode to quantify the electroplated platinum on the carbon felt electrode.
2. Construction and Installation of Two-electrode System
- Investigation of installation site for each electrode in the natural environment.
- Determine oxygen concentration using a dissolved oxygen (DO) probe.
- Check the depth profile of DO in the site.
NOTE: The desired environmental conditions for the anode are consistent hydration and anoxia. If desired, remove the influence of oxygenic photosynthesis by shielding the anode from light. The ideal conditions for cathode placement are consistently hydrated, and near surface waters to be oxic. If necessary, attach floats to maintain surface contact in the cathode.
- Construction of fuel cell type 2-electrode incubation system
- Connect the insulated wire of desired length and a lead of titanium wire from the electrodes (one anode and one platinum plated cathode) by twisting the two lines. Cover the connections with water-resistant wax and further protect using marine grade heat shrink tubes.
- Connect two wires with a cathode and an anode by a resistor of known resistance.
NOTE: For biological systems, lower resistors (10 to 1,000 Ω) result in more consistent biological activity. If desired, a high resistance resistor will prevent biological activity, as a negative control. To prevent a corrosion of any connections between resistor and leads, we protected them with heat shrink tubes.
- Measurement for voltage and temperature logging over time.
- Check the voltage between the ends of the resistor for estimating current production from the fuel cell reaction.
- Measure the voltage difference over time using a data logging voltmeter with the appropriate connections leading to the anode and cathode (see manufacturer's protocol).
NOTE: Simultaneous temperature data logging is optional, but this information can help relate changes in current to abiotic as opposed to biological fluctuations.
- Protection of data logger and electric connections
- Use a stationary and/or plastic bag to protect the logger and all the electric connections from rain.
- Fix the plastic bag and cables tightly to protect from strong wind. An example is shown in Figure 1.
3. Collection of the Electrode Sample from the Natural Environment
- To prevent the quality of the anode sample from being damaged due to oxygen contamination, collect the electrode under anaerobic condition.
- At least 30 min prior to collecting the electrode sample, put a test tube in an anaerobic location. For example, put the test tube and lid separately at the bottom of the pond to make the bottle inside anaerobic.
- Cut the titanium lead from the electrode with a wire cutter, gently collect the electrode sample into the test tube, and seal it in the anaerobic water zone. To keep the sample fresh, store the sample at 4 °C immediately after the sample collection.
NOTE: Alternatively, electrodes can be transferred directly to anoxic (N2 purged) medium. We used a Cedars medium (described by Suzuki et al.11) that was designed from the aqueous geochemistry measured at the site and amended to provide sufficient nutrients for microbial growth. This media was modified for different laboratory experiments.
4. Laboratory Confirmation for Current Production and DNA Analysis
- Electrochemical confirmation for current production capability of microbial consortia attaching to the electrode.
- Construct an electrochemical reactor14,15 with the sampled electrode, a platinum wire, and a Ag/AgCl (KCl saturated) electrode as WE, CE, and RE, respectively, in an anaerobic chamber. Fill the electrochemical reactor with Cedars medium containing soluble carbohydrate electron donors.
- Poise the electrode potential at +0.2 V vs. Ag/AgCl and measure the current production.
- DNA extraction from the electrode sample using a microbial DNA kit (see Table of Materials).
- Clean the inside of anaerobic glove box with 70% ethanol and put a sterilized dish on aluminum foil.
NOTE: Anaerobic chamber keeps oxygen concentration less than 1 ppm by maintaining a hydrogen atmosphere at around ~2–3% to scavenge oxygen in the presence of a palladium catalyst. - Open the electrochemical reactor in the glove box, put the sample electrode on the dish, and cut to a size to fit the tube used in the DNA kit. Proceed with the manufacturer's protocol.
- Clean the inside of anaerobic glove box with 70% ethanol and put a sterilized dish on aluminum foil.
Representative Results
Current production was successfully measured for approximately 3 months using a voltage data logger as shown in Figure 3. This time was chosen as it was the longest stable incubation period for the spring, due to strong fall rains affecting the spring. A shorter period could be sufficient, though a longer period could provide stronger enrichment of biomass. We confirmed the connection of the two-electrode system after electrochemical incubation and observed no evidence of corrosion in the system. Higher current production was observed in the two-electrode system with lower resistance (1,000 Ω) compared with negative control with 100 kΩ resistance. The gradual current production increase in the first month may suggest the growth, accumulation or accommodation of microbes on the surface of the electrode following stable current production for another two months. Interestingly, current production oscillated in an approximately 24 h cycle through the whole period of the electrochemical enrichment.
To confirm the current production capability of microbial consortia attaching on the electrode, we performed chronoamperometry with the collected anode in the laboratory using a 3-electrode electrochemical reactor. We poised the electrode potential at +0.4 V vs. a standard hydrogen electrode (SHE) in the presence of various carbohydrate electron donors. The daily oscillations were no longer observed on the anode when incubated in the laboratory. This suggests that environmental factors impacted the microbial current production, and likely resulted in the observed oscillations.
Comparing the microbial community observed on the enriched electrodes with the attached and planktonic non-electrode communities, we observed distinct differences in the over-arching structure (Figure 4). The electrode microbial community was highly enriched in operational taxonomic units (OTUs) from uncultured lineages, as well as the Firmicute lineages of Bacillus. A shift in the composition of Proteobacteria was also observed; specifically, Betaproteobacteria (predominantly Serpentinamonas sp.) dominated the environmental calcite and planktonic samples, and Gammaproteobacteria dominated the electrode samples10. Differential enrichment of microbial strains between the environment and electrode samples provides support for microbial activity driving the observed experiment. This was further supported through the ultimate isolation of an electrochemically active strain from the enriched Firmictutes OTUs for the Cedars9.
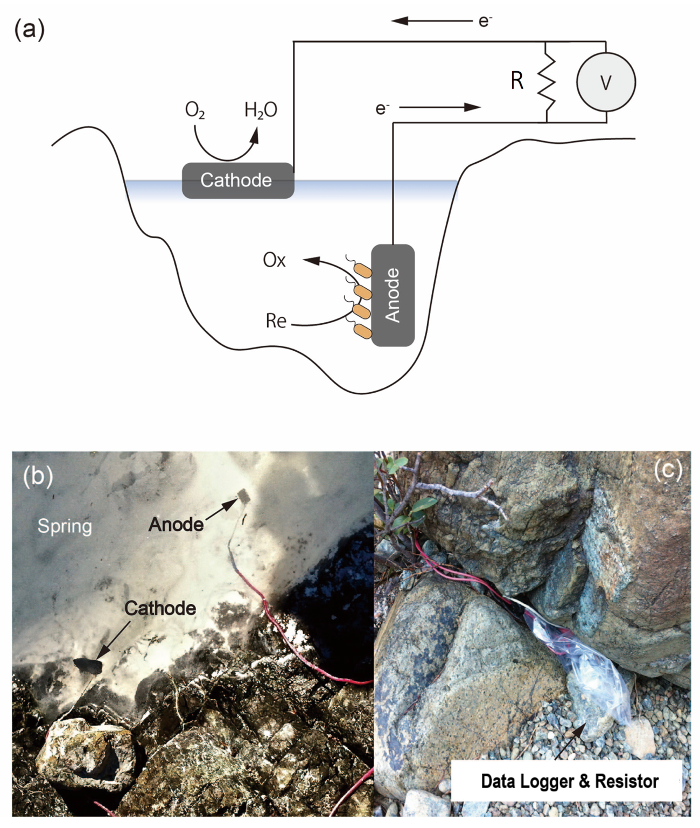
Figure 1: Electrochemical system. (a) Schematic image of the on-site electrochemical system to enrich EET-capable bacteria in the environment. An anode of carbon felt accepts respiratory electrons from the microbe and a cathode of Pt-electroplated carbon felt catalyzes oxygen reduction. Current production was monitored by a data logger V connected in parallel with both ends of a resistor R. (b) Setup example in the Cedars spring where the anode was put at the bottom of spring and cathode near water surface. (c) Protection of data logger and resistor by a plastic bag and a rock. The size of the anode is the same as the one shown in Figure 2. Please click here to view a larger version of this figure.
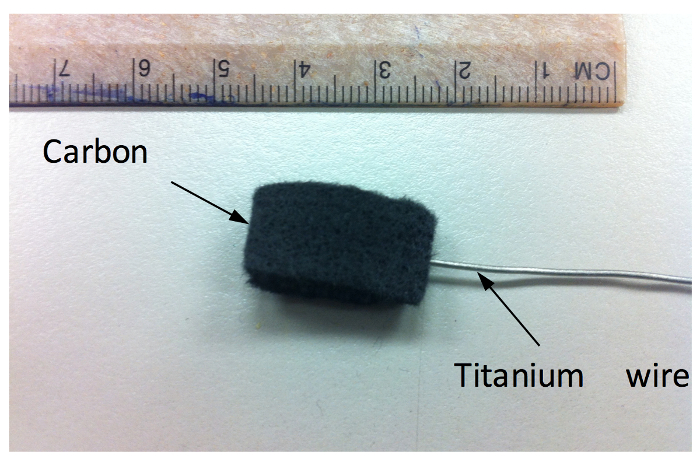
Figure 2: Carbon felt electrode connected to a titanium wire. Please click here to view a larger version of this figure.
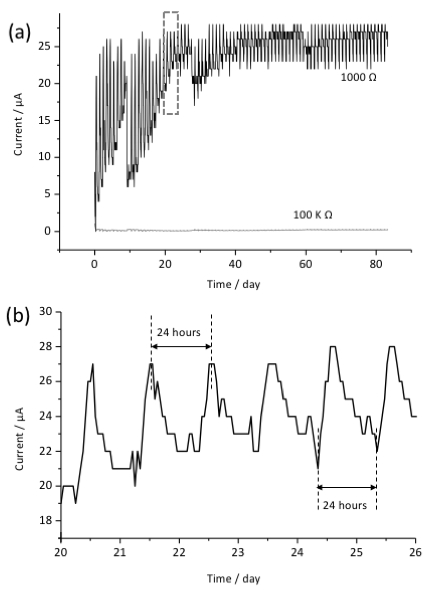
Figure 3: Current production observed in the two-electrode system for a three-month incubation period. Data for systems using resistors of 100 kΩ and 1,000 Ω are shown in (a). Background current was subtracted to zero the initial current value. Panel (b) corresponds to the square in panel (a). Daily current oscillations were observed across the experiments illustrated in panel (a).
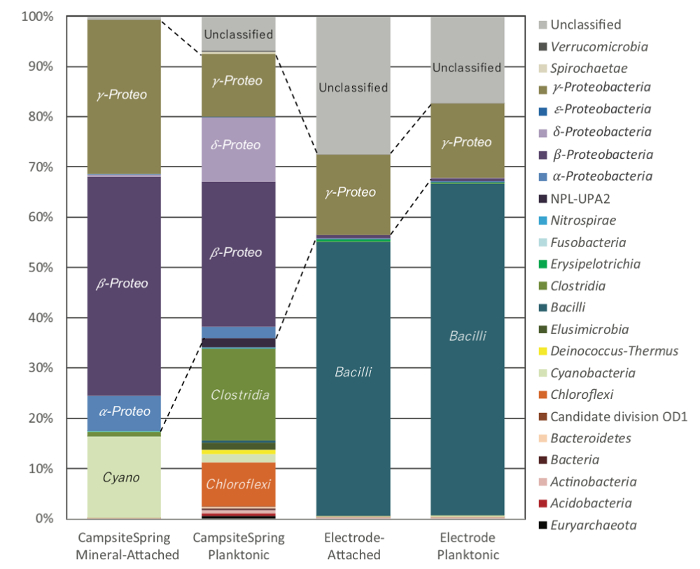
Figure 4: Microbial community sequence distribution for Camp Site springs. DNA extracted from filtered water (CampsiteSpring Planktonic) or 1 g of calcite taken from the pool bottom (CampsiteSpring Calcite Attached) were compared to DNA extracted from carbon felt electrodes (Electrode Attached) or DNA from cells in the fluid phase of the electrochemical reactor (Electrode Planktonic). Sequence designations are based on phylum-level or class-level identities for the dominant phyla Firmicutes and Proteobacteria. Abundances are based on percent total reads. Changes in Proteobacterial lineages are outlined in dotted lines. Please click here to view a larger version of this figure.
Discussion
In the described study, we show the enrichment of a microbial consortium, linked with in situ current production. The observed patterns in current support microbial activity in this system over short and long time scales. The critical step for constructing a functional two-electrode (fuel cell type) system is identifying and utilizing a location with a stable water-level and oxygen concentration in the environment. The cathode is exposed to oxygen at the air water interface, while the anode is kept under anaerobic condition, and the electrode potential difference promotes anaerobic respiration of EET-capable bacteria.
We observed daily current oscillation in the environmental electrochemical system but not in the laboratory reactor. Because this fluctuation of current was observed during the daylight hours-maximum and minimum currents were observed between dawn and dusk-the effect of sunlight and/or temperature could explain the change in the microbial current production. Measuring temperature, sunlight and/or other environmental variables could further expand understanding of the controls and drivers of microbial electron flow in environmental systems. Alternatively, adding elements to block sunlight could help remove or mitigate the effects of oxygenic photosynthesis and/or potential photoreactions on the electrode, which could serve to better stimulate optimal EET conditions. However, measurement of other environmental factors could better elucidate ecological context in EET-capable microbes, including potential microbial community interactions, as well as the relationships between microbes and the environment.
Our two-electrode system potentially enriched not only anode-respiring bacteria, but also oxygen-reducing bacteria that harvest energy from electron uptake. Although we did not conduct the community analysis on the cathode, their microbial electron uptake capability is testable in laboratory three-electrode reactor with negatively poising the collected cathode electrode in the presence of oxygen. A stable concentration gradient of electron acceptors from cathode to anode enable our method to theoretically also enrich cathode-respiring bacteria. An alternative enrichment method for the cathode-respiring bacteria is the use of Fe(0) particles or coupons as a solid electron donor5. Although hydrogen production can also occur at the surface, successful isolation of bacteria that can directly extract electrons from electrode surface has been reported5,16.
In conclusion, our method successfully enriched EET-capable consortia using a self-sustaining electrochemical system in a low-biomass environment. Several previous cultivation approaches were unsuccessful, which led us to develop an on-site enrichment scheme. In our system, current output reflected the microbial activity, and led to further hypotheses about the microbial ecology of this system. Expanding the isolation of EET-capable microbes as well as the diversity of environments will enhance our understanding of the mechanism for EET, as well as the role of electron transport in environmental microbiology.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge Roger Raiche and David McCrory for allowing us access to the Cedars and consulting on locations for long term incubation. We also thank the Cedars field crew during the 2013-2014 season: Shino Suzuki, Shunichi Ishii, Greg Wanger, Grayson Chadwick, Bonita Lam and Matthew Schechter. Additional thanks to Shino Suzuki and Gijs Kuenen for insightful research and culturing support. This work was funded through a Grant-in-Aid for Young Scientists A and B from the Japan Society for Promotion of Science (JSPS) KAKENHI Grant Number 17H04969 and 26810085, respectively, and the Japan Agency for Medical Research and Development (17gm6010002h0002). US funding provided by the US Office of Global Naval Research (N62909-17-1-2038), and the Center for Dark Energy Biosphere Investigations (C-DEBI) (OCE0939564) and the NASA Astrobiology Institute – Life Underground (NAI-LU) (NNA13AA92A). Part of this work was conducted as part of a Japan Society for the Promotion of Sciences: Short-term postdoctoral fellowship for Annette Rowe (PE15019) at the University of Tokyo in the lab of Kazuhito Hashimoto.
Materials
| Carbon felt sheet | n/a | n/a | Used for anode and cathode |
| Titanium wire | The Nilaco Cooporation | TI-451485 | Used to construct fuel cell system |
| Graphite epoxy | Electrolytica lnc. | n/a | Used to connect the electrodes and Ti wire |
| Drying oven | Yamato | DY300 | bake the electrode to solidify conductive graphite epoxy |
| Digital multi meter | Fluke | 616-1454 | to check the ohmic value of resistance |
| Dissolved oxygen probe | Sper Science | # 850045 | to check the oxygen concentration in the environments |
| Resistor | Sodial | Used to construct fuel cell system |
|
| Conducting wire | Pico | 81141s | Used to construct fuel cell system |
| Voltmeter and Data logger | T&D corporation | VR-71 | Used for data recording |
| Hydrogen Hexachloroplatinate(IV) Hexahydrate | wako | 18497-13-7 | Used for electropolation |
| Citric acid | Wako | 038-06925 | Used for electropolation |
| Sulfuric acid | Wako | 192-04696 | Used for electropolation |
| HCl | Wako | 083-01095 | Used for electrode washing |
| Glass cylinder | N/A | N/A | Custom-made, used as the electrochemical reactor |
| PTFE cover and base | N/A | N/A | Custom-made, used as a cover and a foundation of the electrochemical reactor |
| Buthyl rubber | N/A | N/A | Custom-made, inserted between each component of electrochemical reactor |
| Septa | GL Science | 3007-16101 | Used as an injection port of electrochemical reactor |
| Indium tin-doped oxide (ITO) electrode | GEOMATEC | No.0001 | Used as a working electrode, 5Ω/sq |
| Ag/AgCl KCl saturated electrode | HOKUTO DENKO | HX-R5 | Used as a reference electrode, Φ0.30mm |
| Platinum wire | The Nilaco Cooporation | PT-351325 | Used as a counter electrode |
| NaHCO3 | Wako | 191-01305 | Used for The Cedars Media (CMS) |
| CaCO3 | Wako | 030-00385 | Used for CMS |
| NH4Cl | Wako | 011-03015 | Used for CMS |
| MgCl2 • 6H2O | Wako | 135-00165 | Used for CMS |
| NaOH | Wako | 198-13765 | Used for CMS |
| Na2SO4 | Wako | 194-03355 | Used for CMS |
| K2HPO4 | Wako | 164-04295 | Used for CMS |
| CABS | SANTA CRUZ | SC-285279 | Used for CMS |
| Incubator | TOKYO RIKAKIKAI CO. LTD. | LTI-601SD | Used for precultivation |
| Autoclave machine | TOMY SEIKO CO. LTD. | LSX-500 | Used for sterilization of the electrochemical reactor and the medium |
| Clean bench | SANYO | MCV-91BNF | Used to prevent the contamination of the electrochemical reactor and the medium with other microbes |
| Centrifuge separator | Eppendorf | 5430R | Rotational speed upto 6000×g is required |
| Nitrogen gas generator | Puequ CO. LTD. | PNTN-2 | Nitrogen gas cylinder can also be used instead of gas generator |
| UV-vis spectrometer | SHIMADZU | UV-1800 | Used for optimization of cell density |
| Potentiostat | BioLogic | VMP3 | Used for biofilm formation and kinetic isotope effect experiments |
| Thermal water circulator | AS ONE | TR-1A | Used for maintanance of temperature of electrochemcial reactor |
| Faraday cage | HOKUTO DENKO | HS-201S | Used for electrochemical experiments |
| Anaerobic Chamber | COY | TypeB (Vinyl) | TO conduct experiments under anaerobic condition |
| Ultraclean DNA Extraction kit | MoBio |
Referenzen
- Nealson, K. H., Saffarini, D. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annual Reviews of Microbiology. 48, 311-343 (1994).
- Lovley, D. R. Bug juice: harvesting electricity with microorganisms. Nature Reviews Microbiology. 4 (7), 497-508 (2006).
- Rabaey, K., Rozendal, R. A. Microbial electrosynthesis – revisiting the electrical route for microbial production. Nature Reviews Microbiology. 8 (10), 706-716 (2010).
- Lovley, D. R., Coates, J. D. Bioremediation of metal contamination. Current Opinion in Biotechnology. 8 (3), 285-289 (1997).
- Dinh, H. T., et al. Iron corrosion by novel anaerobic microorganisms. Nature. 427 (6977), 829-832 (2004).
- Myers, C. R., Nealson, K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 240 (4857), 1319-1321 (1988).
- Lovley, D. R., Phillips, E. J. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Applied and Environmental Microbiology. 54 (6), 1472-1480 (1988).
- Arnold, R. G., DiChristina, T. J., Hoffmann, M. R. Reductive dissolution of Fe(III) oxides by Pseudomonas sp 200. Biotechnology and Bioengineering. 32 (9), 1081-1096 (1988).
- Rowe, A. R., et al. In situ electrochemical enrichment and isolation of a magnetite-reducing bacterium from a high pH serpentinizing spring. Environmentakl Microbiology. 19 (6), 2272-2285 (2017).
- Suzuki, S., et al. Microbial diversity in The Cedars, an ultrabasic, ultrareducing, and low salinity serpentinizing ecosystem. Proceedings of the National Academy of Science U S A. 110 (38), 15336-15341 (2013).
- Suzuki, S., et al. Physiological and genomic features of highly alkaliphilic hydrogen-utilizing Betaproteobacteria from a continental serpentinizing site. Nature Communications. 5, 3900 (2014).
- McCollom, T. M., et al. Temperature trends for reaction rates, hydrogen generation, and partitioning of iron during experimental serpentinization of olivine. Geochimica et Cosmochimica Acta. 181, 175-200 (2016).
- Morrill, P. L., et al. Geochemistry and geobiology of a present-day serpentinization site in California: The Cedars. Geochimica et Cosmochimica Acta. 109, 222-240 (2013).
- Okamoto, A., Nakamura, R., Hashimoto, K. In-vivo identification of direct electron transfer from Shewanella oneidensis MR-1 to electrodes via outer-membrane OmcA-MtrCAB protein complexes. Electrochimica Acta. 56 (16), 5526-5531 (2011).
- Okamoto, A., Hashimoto, K., Nakamura, R. . Spectroelectrochemical Investigation on Biological Electron Transfer Associated with Anode Performance in Microbial Fuel Cells. , 207-222 (2012).
- Deng, X., Nakamura, R., Hashimoto, K., Okamoto, A. Electron from an Extracellular Electrode by Desulfovibrio ferrophilus Strain IS5 Without Using Hydrogen as an Electron Carrier. Electrochemistry. 83 (7), 529-531 (2015).

