10.1:
VSEPR Theory and the Basic Shapes
59,008 Views
•
•
Overview of VSEPR Theory
Valence shell electron-pair repulsion theory (VSEPR theory) enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an arrangement that minimizes repulsions between these electron pairs by maximizing the distance between them. The electrons in the valence shell of a central atom form either bonding pairs of electrons, located primarily between bonded atoms, or lone pairs. The electrostatic repulsion of these electrons is reduced when the various regions of high electron density assume positions as far from each other as possible.
VSEPR theory predicts the arrangement of electron pairs around each central atom and, usually, the correct arrangement of atoms in a molecule. We should understand, however, that the theory only considers electron-pair repulsions. Other interactions, such as nuclear-nuclear repulsions and nuclear-electron attractions, are also involved in the final arrangement that atoms adopt in a particular molecular structure.
Application of VSEPR Theory
VSEPR theory can be used to predict the structure of molecules. For example, let us predict the structure of a gaseous CO2 molecule. The Lewis structure of CO2 (Figure 1) shows only two electron groups around the central carbon atom. With two bonding groups and no lone pairs of electrons on the central atom, the bonds are as far apart as possible, and the electrostatic repulsion between these regions of high electron density is reduced to a minimum when they are on opposite sides of the central atom. The bond angle is 180°.
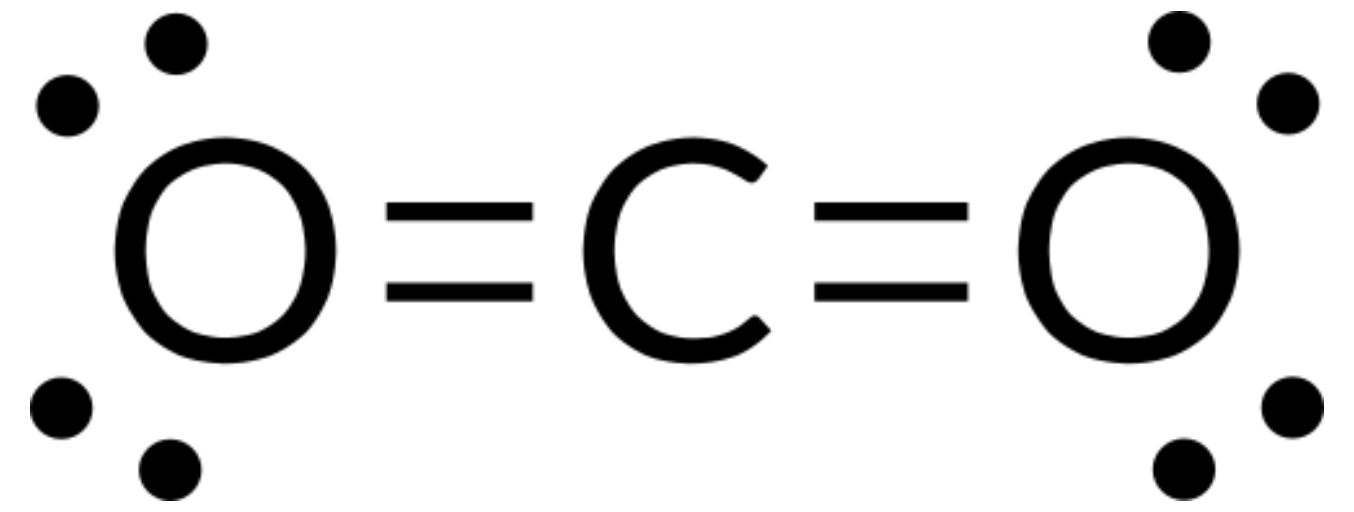
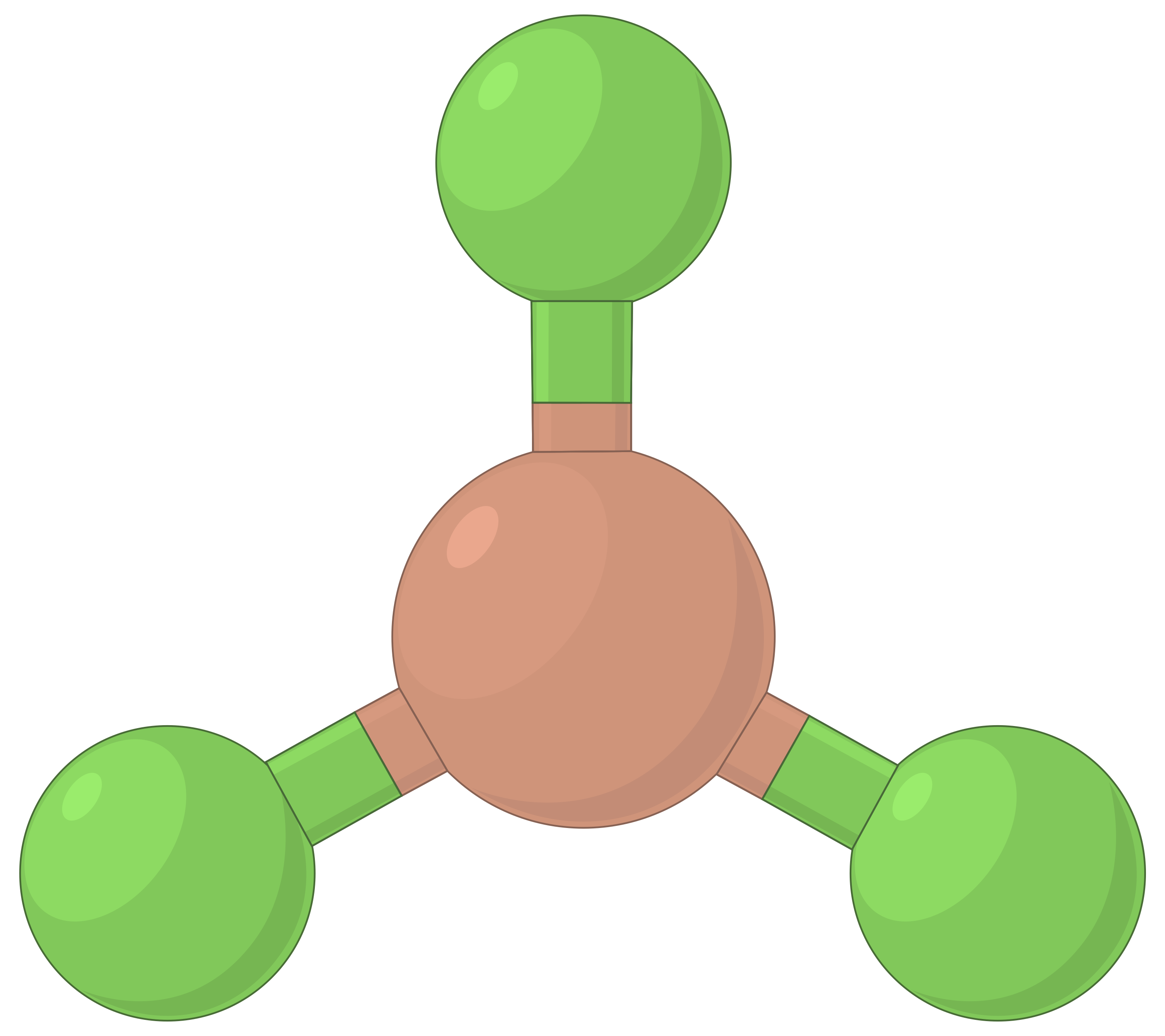
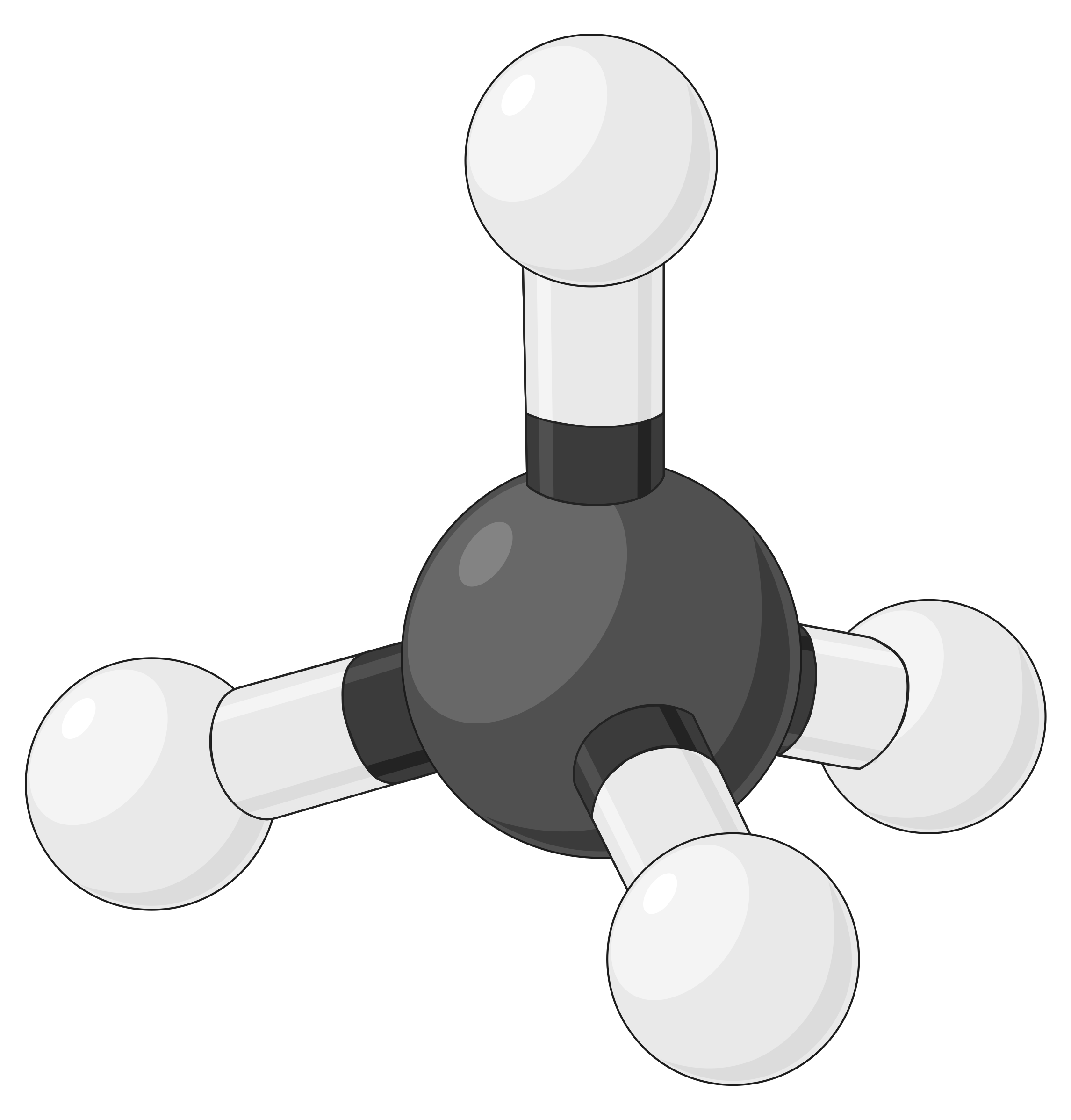
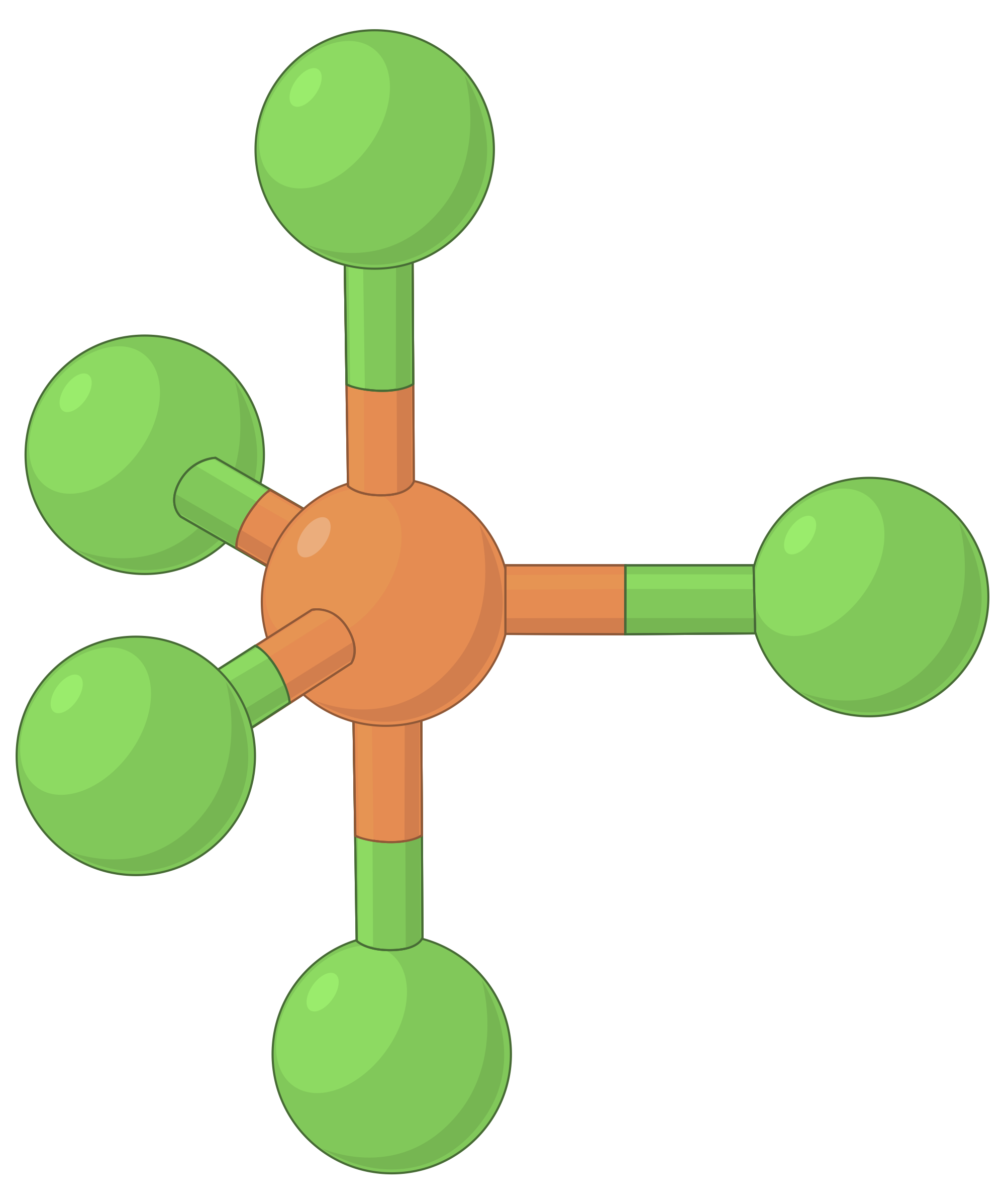
The table below illustrates electron-pair geometries that minimize the repulsions among regions of high electron density (bonds and/or lone pairs). Two regions of electron density around a central atom in a molecule form a linear geometry; three regions form a trigonal planar geometry; four regions form a tetrahedral geometry; five regions form a trigonal bipyramidal geometry, and six regions form an octahedral geometry.
| BeF2 | BF3 | CH4 | PCl5 | SF6 | |
| Number of electron regions | 2 | 3 | 4 | 5 | 6 |
| Electron region geometry | Linear; 180° angle | Trigonal planar; all angles 120° | Tetrahedral; all angles 109.5° | Trigonal bipyramidal, angles 90° or 120°. | Octahedral; all angles 90° or 180°. |
| Spatial arrangement | 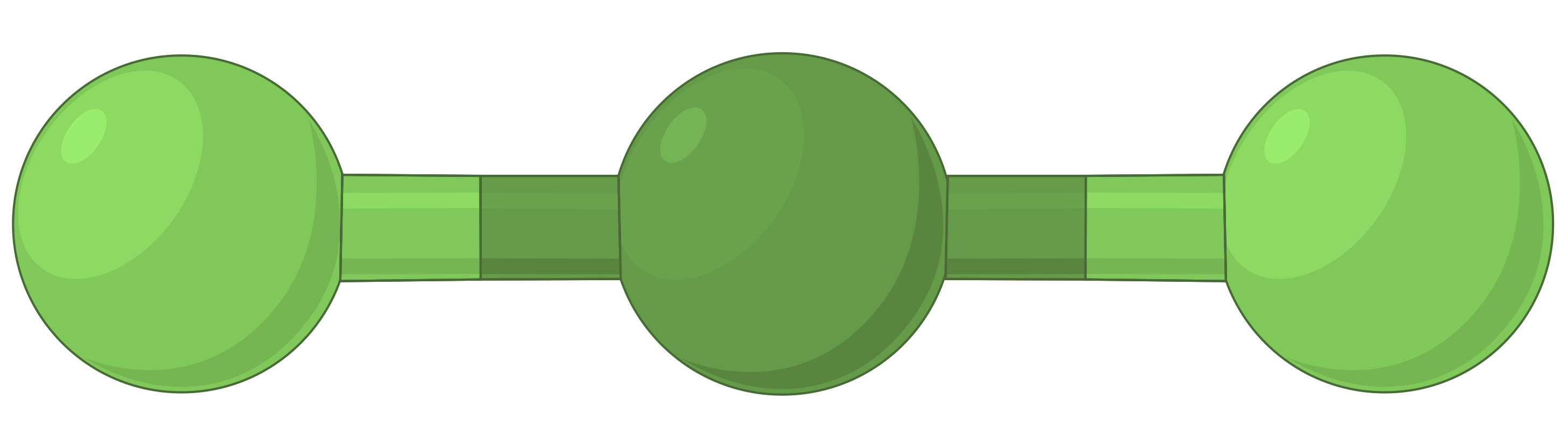 |
 |
 |
 |
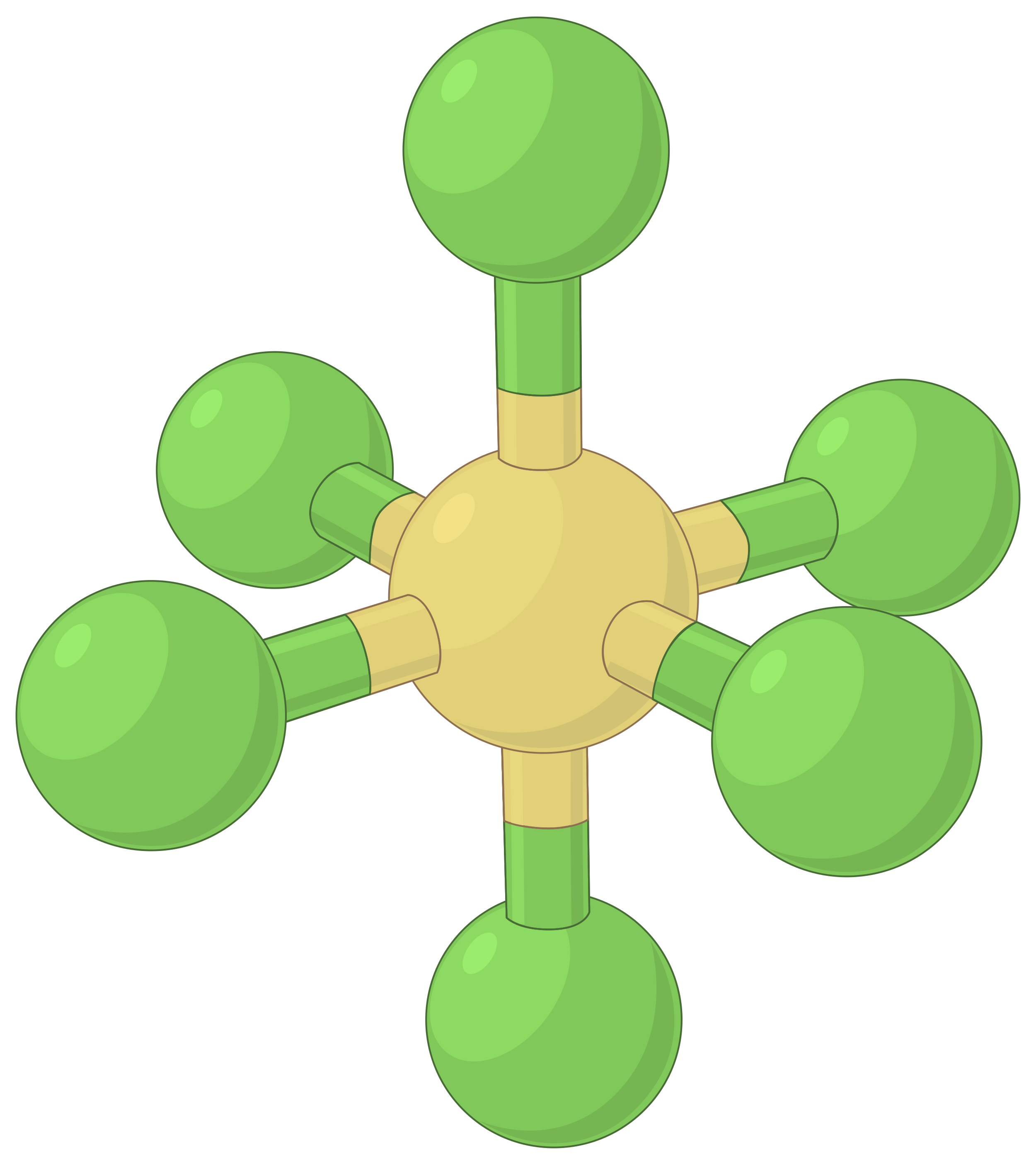 |
Table 1. The basic electron-pair geometries predicted by VSEPR theory maximize the space around any region of electron density (bonds or lone pairs).
This text has been adapted from Openstax, Chemistry 2e, Section 7.6: Molecular Structure and Polarity.
