Behavioral Training Procedures for Head-fixed Virtual Reality in Mice
Summary
The article describes the experimental procedures for the commonly used linear track virtual reality (VR) paradigm in mice as well as determining the feasibility of running complex VR tasks by testing a Y-shaped signal discrimination task.
Abstract
Virtual reality (VR) combined with head-fixation is increasingly being utilized in behavioral neuroscience studies as it allows complex behavioral assays to be performed in head-fixed mice. This enables precise behavioral recordings while incorporating various neurophysiological techniques that require head-fixation to minimize movement-related signal noise during neural recordings. However, despite the growing use of VR, there is little published data on the detailed methodology of how to implement it. In this study, a training protocol is developed whereby male and female C57B16/J mice are trained to run down a virtual linear corridor, the length of which is increased from 1-3 m over multiple training sessions. Building upon this foundation, this study investigated the feasibility of mice performing complex behaviors within VR using a Y-maze paradigm. The task required navigating to the arm with black walls from the choice point in the Y-maze. After reaching a criterion of two consecutive days equal to or greater than 70% correct, the mice progressed to increasingly difficult sensory discrimination. The findings provide important details on the methodologies useful for the successful training of mice in VR and demonstrate that mice exhibit learning capabilities in navigating the Y-maze. The methodology presented not only offers insights into training duration in VR-based assays but also underscores the potential for probing intricate behaviors in mice, opening avenues for more comprehensive neuroscience investigations.
Introduction
Virtual reality tasks have emerged as a powerful method of behavioral assessment in mice due to head-fixation, which allows for mechanical stability that would be compromised in freely behaving mice1. This method enables reduced movement artifacts in electrophysiological recordings2,3 and optical imaging4,5,6,7. It also facilitates repeatable behaviors8 and precise eye-tracking9. In the experimental setup, the mouse is fixed in place and situated atop an air-supported spherical treadmill. This apparatus allows for the intricate exploration of visually guided behavior within the VR environment. As the mouse moves on the treadmill, its locomotion synchronizes seamlessly with its navigation within the virtual landscape, which is visually depicted on the screen surrounding the mouse.
The aim of this study is two-fold: to address key challenges within experimental behavioral neuroscience and to contribute to the advancement of methodologies in this field. Firstly, despite the increased use of VR in academic research10,11,12, there remains a notable absence of comprehensive methodologies and training protocols, hindering the adoption of this technology by new investigators. The primary goal was to fill this gap by delineating a detailed training regimen for the linear track paradigm, as depicted in prior studies13,14,15. A commercially available system is used to describe these operational procedures. As a disclaimer, these procedural guidelines have components specific to this system; however, for a discussion of the generalizability of this protocol, see the discussion. The objective was to outline the behavioral procedures, the typical timeline for performing these procedures, and the success rate for training mice to run on a simple linear track.
Second, there remains a lack of documentation on the implementation of complex maze tasks within this paradigm in mice. Complex virtual assays have been developed in rats11. However, mice have reduced visual acuity in comparison16 and often perform worse in complex tasks17. While some investigations have focused on specific tasks such as evidence accumulation or spatial novelty18, the focus here was in elucidating the training methodologies required for mice to engage in decision-making paradigms within VR environments. To address this challenge, a signal discrimination task was devised where the mice were tasked solely with learning to associate the color/luminance (black versus white) of the rewarded arm with the reward, achieved by selecting the black arm at the choice point of the Y-maze, with the correct arm randomized on each trial. This task was designed to require interaction with the virtual cues and provide insight into the perceptual discrimination abilities of the mice.
In summary, this study addresses critical gaps in the field of experimental behavioral neuroscience by providing comprehensive training protocols for using VR paradigms in mice and elucidating methodologies for complex decision-making tasks within this framework. By leveraging insights from previous research and innovative experimental designs, this study aims to streamline research practices and advance the understanding of neural mechanisms underlying behavior. The following sections will delve deeper into the experimental procedures and results and discuss the findings.
Protocol
All procedures involving animals were conducted in strict adherence to the protocols established by the NIEHS Animal Care and Use Committee, ensuring compliance with ethical standards and welfare guidelines. C57BL/6Tac mice, approximately 8 weeks old, were utilized for the study.
1. Surgery for head-bar implantation
- Surgery preparation
- Obtain the desired quantity of mice for one's cohort, ideally housing them individually to minimize interference with the head-bar implant, although this is optional19. This study used a sample size of three male and three female mice (initially balanced, but one male was excluded early in training after failing to run on the ball)
- Acquire the materials specified in the Table of Materials, adjusting according to the specifics of the study design.
- Upon acquisition of the mice, designate individual identifiers and apply either tail tattoos or ear hole punches to ensure unambiguous identification. Establish a comprehensive log to systematically record their weights as required for the water restriction procedure.
- Anesthesia administration
- Ensure that all surgical instruments are readily available, including appropriate syringes, a heating pad, metalware (such as tweezers, micro scissors, and hemostats), iodine solution, eye lubricant, and beakers for saline and hydrogen peroxide. To ensure sterile conditions, sanitize all surgical equipment and sterilize all surgical tools using an autoclave.
- Before proceeding with surgery, conduct precise weight measurements of the mice and activate the heating pad to 34 °C. Record all necessary information in one's institution's laboratory/surgical notebook. Verify the adequacy of oxygen and isoflurane tank levels and confirm the availability of all essential materials to facilitate uninterrupted surgical procedures.
- Scruff the mouse and place it into a nosecone attached to a vaporizer designed for small animals receiving 4% isoflurane and an oxygen flow rate of 3 L/min to induce anesthesia. Use a scavenger to capture any potentially harmful waste gases (recommended).
- Administer Petroleum ophthalmic veterinary ointment to the mouse's eyes while it is under the nosecone to prevent ocular dryness. Apply a single drop to each eye initially and reapply as necessary. Ensure constant protection of the eyes by always maintaining a layer of this lubricant with periodic checking.
- Prepare the surgical site (Figure 1A) on the mouse's head by shaving the area where the cranium will be affixed to the head-bar.
- Position the mouse's incisors within the stereotaxic apparatus beneath the nosecone, adjusting the oxygen flow rate to 1 L/min and administering 1%-2% isoflurane from the vaporizer. Ensure proper anesthesia flow by adjusting the valves and switching between the induction nosecone and the stereotaxic apparatus accordingly. Extend the mouse's hind leg and apply firm pressure to the toe. If the foot does not exhibit a reflexive withdrawal response, this indicates that the anesthesia is effective. Repeat every 15 min, along with a breathing check.
- Secure the mouse's head in place by attaching surgical stability bars within its ear canals, minimizing any potential head movement during surgery.
- Prior to making incisions or injections, sterilize the shaved surgical area on the top of the head by scrubbing it with a swab dipped in an iodine antiseptic. From this stage onward, employ sterile gloves to maintain aseptic conditions.
- Injection administration
- Subcutaneously inject 0.05 mL of bupivacaine (local analgesia) with a 25G needle into the surgical incision site on the scalp.
- Subcutaneously inject 1 mL of saline (hydration) with a 25G needle into one side of the interscapular region.
- Subcutaneously inject 0.05 mL of buprenorphine (whole-body analgesia) with a 25G needle into the opposite side of the interscapular region.
- Exposing the skull
- Use micro scissors to create an incision of the skin above the interfrontal and internasal sutures of the cranium, starting from just above the eyebrow ridge and extending to behind the occipital notch (Figure 1A).
- Use hemostats to hold down the left and right flaps of the skin, exposing the cranium.
- Use a dry cotton swab to remove connective tissue from the scalp between the pinned-back skin folds.
- Use a cotton swab wetted (but not saturated) with hydrogen peroxide to scrub the scalp, ensuring visibility of the sutures and being careful not to get hydrogen peroxide on the surrounding tissue.
- Repeat steps 1.4.3 and 1.4.4, 2x-3x, until both bregma and lambda are distinctly visible, and the scalp is thoroughly cleaned.
- Surgical screw implantation
- Attach two screws to the skull, positioning one screw posterior to bregma and the other anterior to lambda (Figure 1B) to maximize surface area for dental adhesive and increase the stability of the head bar. Position the location of the screws at targets a specified distance from bregma. Ensure that one screw is positioned on the left and the other on the right (i.e., Anterior-Posterior (AP) +1.00, Medial-Lateral (ML) -1.00 and AP -3.00, ML +3.00), ensuring there is adequate space between the screws to accommodate the placement of the head-bar and adjust the coordinates and needed.
- Drill target positions, ensuring that drilling is limited to the bone of the cranium and does not penetrate the brain tissue.
- Using a screwdriver, screw roughly half of the screw into place. Repeat for the second screw.
- Attaching the head-bar implant
- Blend dental cement and administer it to the underside of the head-bar, focusing on the concave surface, and apply along the interfrontal suture of the cranium.
- Position the head-bar over the interfrontal suture to facilitate bonding between the dental cement on the head-bar and that on the suture. Securely hold it in place by hand at the desired angle for approximately 5 min until it sets. Apply additional dental cement as needed. (Figure 1C-E)
- Reattaching the skin over the head-bar
- Release the hemostats and utilize tweezers to reunite the two skin flaps over the dried dental cement-fixed head-bar. Use topical tissue adhesive to delicately secure the skin together by slowly adhering the left and right portions of the scalp together over the head-bar, starting from the anterior incision site and ending at the posterior incision site.
- Allow the topical tissue adhesive to harden to confirm the surgical area has been resealed before releasing the mouse from the surgical stability bars and nosecone.
- Transfer the mouse to a singly housed cage and place it on a heating pad at 37.5 °C.
- Carefully monitor the mouse for any signs of discomfort or breathing irregularities until it has regained consciousness. Do not leave the mouse unattended until it has regained sternal recumbency, exhibits alertness, and is ambulatory.
- Following surgical procedures, allow mice to undergo a 1-week rest period. Monitor the mice daily to detect and address any notable fluctuations in weight. Provide mash for the mice 3 days post-surgery to aid in recovery. To prevent interference with the head-bar, house these mice individually.
2. Fluid restriction
NOTE: Water restriction induces a state of thirst in mice, heightening their motivation for liquid rewards. However, meticulous implementation is necessary to ensure the preservation of mouse well-being20.
- At 1 week from the surgery day, establish baseline weights for mice.
- Tape one segment of a small Petri dish (60 mm x 15 mm) concave-side down onto the cage floor, taping a smaller Petri dish (35 mm x 10 mm) concave down to the center of the flat surface of the Petri dish taped to the floor, with another small Petri dish (60 mm x 15 mm) concave up taped atop the middle dish's flat surface to serve as a water reservoir (Figure 2).
- Ensure that the height of the upper dish prevents contamination from bedding material while allowing mice easy access to water. Add the daily water allowance volume to the reservoir using a pipette.
- On day 1, provide mice with a dose of 15 ml water per 100 g of body mass.
- On day 2, provide mice with a dose of 10 mL water per 100 g of body mass.
- On day 3, provide mice with a dose of 5 mL water per 100 g of body mass. Mice should receive a minimum intake of 1 mL of water per day throughout the study, regardless of body weight.
NOTE: Researchers may opt to administer the minimal volume uniformly across all subjects, although such adjustments should be made with careful consideration. - Keep a consistent dosage throughout the study duration of water allocation at 5 mL per 100 g body weight (or a uniform 1 mL water allocation if preferred)
NOTE: Mice should be provided ad libitum access to water for 1-2 days per week when mice are not performing a VR experiment (i.e., during the weekend). This will facilitate the restoration of their natural hydration levels. In instances where mice fall below 90% of their recorded baseline weights, they should be transitioned to ad libitum water access until they reach 90% of their baseline weight. Mice falling below 80% of their recorded baseline weights should be ethically euthanized. - Delay administering their daily water dose at least 30 min post-behavioral assessment to mitigate potential interference with their natural thirst behaviors essential for conducting the experiment accurately.
NOTE: Providing liquid rewards immediately after a trial may inadvertently influence the mice's performance, as they may anticipate receiving an immediate reward, potentially compromising task engagement. Therefore, delaying access to water post-trial prevents habituation to immediate reward delivery and preserves the integrity of the experimental setup.
3. System setup
- Familiarization with equipment: For the hardware components and other considerations of the VR behavioral systems see the steps below.
See discussion section for an examination of protocol generalizability to comparable system setups.- Fully immersive virtual display or dome: This virtual display provides complete immersion for an animal within a customizable virtual environment. The motion within the virtual environment is synchronized with the movement on the spherical treadmill.
- Liquid reward system: The liquid reward system functions through the delivery of liquid reinforcement (water or sugar water) using a peristaltic pump, which directs the reward solution through a plastic-coated metallic tube extending to the mouse when a task is successfully executed. It contains sensors that monitor the quantity of rewards obtained by a mouse during a trial.
- Clean the reward tube weekly using ethyl alcohol or an alternative cleaning agent. To do this, flush 2-5 mL of the cleaning agent through the tube, similar to the delivery of liquid reward, followed by a comparable flush with an equal volume of water.
- When starting the experiment, determine the dispensing rate of the liquid reward from the reward tube by activating it for a specified duration and measuring the volume of dispensed liquid. This procedure allows determination of the peristaltic pump's liquid delivery rate. In this investigation, a dispensing rate of approximately 0.0083 mL/s was used.
NOTE: Most systems offer programmable settings for the duration between behavior execution and reward release, enabling precise planning of the study protocol based on the intended reward volume per trial. The quantity used was determined to be sufficient as it allowed adequate time for the mouse to consume the reward, and its volume appeared to be motivating. - Some protocols may require the mouse to lick the reward spout in order to initiate reward delivery. For the type of tasks employed here this functionality was not employed, deliver rewards contingent solely on successful execution of the desired behavior (i.e. choosing the correct arm in the y-maze). This helps to avoid failures early in training where the tendency to lick has not been sufficiently established, and the initial lick is less likely to occur. It also can allow for measurement of the reward expectancy, which is dissociable from navigational performance under some conditions11.
- While some experiments using fluid restriction opt for the use of standard water through the reward tube, here use sugar water (10% sucrose v/v) as an additional motivational stimulus within the operant paradigm. Notably, enhanced performance across multiple experimental cohorts was observed with the introduction of sugar water.
- Styrofoam ball: This ball acts as a spherical treadmill. When cushioned with air from below, train the mice to run or walk comfortably on the ball. Position it atop a ball holder equipped with motion tracking sensors which collect data on distance and velocity.
- Head holder: Position the apparatus posterior to the mouse, ensuring visual alignment with the VR display upon attachment of the head bar to the holder. This apparatus is crucial for maintaining the mouse in a head-fixed position, thereby mitigating motion artifacts, particularly when the system is utilized alongside optical imaging or electrophysiological techniques.
- Air flow hardware: Configure the airflow from a compressed air source to the ball for the creation of a weightless environment conducive to mice running on the ball. This setup requires a flow regulator to ensure precise control over the air pressure applied to the ball. The ball operates efficiently within the weightless environment with minimal air supply. Therefore, during system setup, ascertain the minimum quantity of air necessary to facilitate smooth and unhindered movement of the ball within the holder. A flow between 10-20 L/min is recommended.
- Software setup: For specific details for the operation of the system, see below.
NOTE: Much like the design of video games21, the architecture of virtual worlds integrates key elements such as an external controller, a programmable navigable environment, and a schedule file containing a state diagram delineating dynamic functions. These components synergistically converge to craft a cohesive interactive experience for subjects engaged in research studies. The operational efficacy of the software depends on precise file organization within designated folders. This explanation will outline the main steps needed to populate pre-made templates, allowing for easy adjustments to existing files and saving them as new versions. These new versions will then form the basis of the study.- Use the following three files together to configure an operational virtual landscape.
- XML files: This file format provides users with the capability to manipulate the photo texture of various elements such as the sky, floor, and walls. Place the files used for the imagery in the Data subfolder of the VR folder. Using these, specify the dimensions of the maze and determine the initial position of the mouse within the maze. Define 3D objects (visual cues) at certain nodes within the maze using these files. Modify these files using a text editor.
- XLSX files: These operate as the command files that configure all three file types (XML, XLSX, and XAML) together to form a cohesive and interactive virtual presentation. Use these files to define the experimental routines that run the VR and its accessories, such as gain sensitivity, which data is extracted, and which files are grouped together for an experiment.
- XAML files: The software application provides a graphical interface for the creation of experimental schedules through the utilization of flowcharts. It facilitates the definition of temporal parameters for the trial, controls for teleportation after the trial is completed, and the activation timing for digital outputs within the trial framework.
- Use the following applications for the acquisition of data and user control of the system while it is in operation.
- [application] VR: Associated with the .XML file that shows the representative landscape, open the file to preview the virtual landscape on the monitors in static mode. For dynamic interaction, open the paired configuration. XLSX file in the control application.
- [application] control: Associated with the .XSLX file, open this application to see the accessory devices associated with the system. One can manually extend and retract the reward tube, dispense liquid reward, and view real-time data acquisition from here.
- [application] schedule designer: This application provides the capability to adjust XAML files to establish a schedule for event triggering within the experiment. For example, design a customizable trigger to determine the duration for dispensing rewards and define the duration of breaks between trials for mice.
- Use the following three files together to configure an operational virtual landscape.
- Example Startup: Begin by deciding what the study protocol will look like based on the adjustable components from steps 3.2.1.1-3.2.1.3. After an operant protocol is clearly defined, open one of the template experiments that came preset with the VR system by following the steps below.
- Open the VR application, which opens to the Data subfolder. Save the virtual landscape created as an XML file. Open this file, and the virtual landscape should appear on the VR monitors.
- Open the Control application and navigate to the Open folder icon in the top right of the screen. Click the Icon, which should bring up the Configs folder, where the corresponding .XLSX experimental configuration is located. Open the .XLSX file with the same name as the .XML file opened in the VR application. The defined system accessories, like the pump and motor for the extendable reward device, are now visible under the control tab within the application.
- Initiate the experimental trial, as the coordination between these two applications enables the creation of an interactive virtual landscape. Ultimately, this integration facilitates the monitoring of essential data, including distance in the XY plane and the collection of rewards with timestamps.
- Data acquisition: Extract the most valuable behavioral data from the system, which are timestamped positional data and rewards. These data are saved separately as log files.
- Position data: To acquire this, follow the steps described below.
- To acquire timestamped XY positional data of the mice, first open the spreadsheet file of the maze of desired data acquisition. In table 1, put the command WriteVRAndCamInfoToFile in one of the cells below the others in column A. Now, positional data will be saved as a dated CSV file (named Log files-MM.DD.YYYY_VRandPathPos.csv) automatically into the configs folder after a trial.
- To export the position data after a trial, close the control application, and the data will be saved to a dated CSV file. This file will contain all the specific data for a particular day, so be careful to manually note when each subject was placed on and taken off the ball. Open the file and import it using the Unicode UTF-8 character set. Column A is labeled DateTime, right click on the A tab and click Format Cells. Go to time and click the MM/DD/YYYY HH:MM: SS option. Now, each system event will be cataloged chronologically for further data analysis.
- Reward data: To acquire this, follow the steps described below.
- Data on pump activation (reward dispensing) are automatically saved as dated log files in the system, so there is no need to put in a command like one would for the position data. To access these, go to the Log Files subfolder of the configs folder.
- Repeat step 3.4.1 for position data for the reward data to export the data as a spreadsheet file. Open the configs folder and select the dated reward file (named Corridor- MM.DD.YYYY or Corridor_Linear_Run- MM.DD.YYYY) when viewed in the folder. This will provide the date and time for when the mice acquired the rewards, and one can use this in further data analysis depending on the paradigm they employed.
- Position data: To acquire this, follow the steps described below.
4. Behavioral Tasks
NOTE: In accordance with established methodologies in behavioral neuroscience, the formulated tasks employ a reward-based associative learning technique. By employing immediate rewards to reinforce specific behaviors, animals are trained effectively to execute repetitive tasks, facilitated by the teleportation capability of VR. Within a virtual behavioral framework, teleportation functionality affords mice the ability to engage in tasks without the stress associated with physical manipulation, concurrently reducing the setup duration necessary for analogous real-world tasks. During the training sessions, use dim red overhead lighting within the experimental setting. This precaution is recommended due to the diminished visual perceptive sensitivity in mice to red light, which mitigates potential interference with their perception of the virtual reality (VR) screens, as opposed to the use of white light22.
- Habituation
- Start the habituation to the spherical treadmill at the same time as their habituation to fluid regulation to associate the lick tube with the reward using the correctly timed physiological motivation. A three-day habituation period is recommended prior to starting the linear track training.
- On the 1st day, handle the mice for 5 min following weighing. During this interaction, it is advised to gently grasp the head-bar implant while the mice are in their cage, creating familiarity with such manipulation. Introduce them to the area where the VR is housed on this day to allow them to anticipate the spatial environment in which experimental trials will occur. This initial day of habituation coincides with the initiation of fluid regulation of 15 mL per 100 mg of body mass.
- On the 2nd day, which aligns with the transition to the 10 mL per 100 mg of body mass fluid regulation step, again handle the mice for 5 min. Continue repeated gentle grasping of the head-bar while in the cage. Affix the head bar to the holder while allowing mice to familiarize themselves with the spherical treadmill for 5-20 min, either on an infinitely repeating track or without the software program activated. This facilitates their adaptation to the head-fixed condition. It should be anticipated that mice may excrete waste during this period, which typically diminishes over successive sessions.
- On the 3rd day, which corresponds to the final day of the fluid regulation paradigm (5 mL per 100 mg of body mass), handle the mice for 5 min. Then, securely attach them to the air-cushioned spherical treadmill and introduce them to liquid rewards through the reward tube.
- Introducing the lick spout to naïve mice will confuse them at first, so make sure the mouse is aware that they are supposed to drink from the tube.
- Without being too forceful, apply the positioning of the mouse guidelines below and individualize the positioning of the mouse on the ball with respect to the tube in a way that will deliver the reward to them in a comfortable manner. When starting, make sure the mice are drinking from the tube; this will occur in most mice naturally under water-restricted conditions when presented with liquid to drink.
- Positioning the mice
- Pre-positioning: Prior to placing the mouse on the ball, extend the centered reward tube with a small droplet of reward at the tip. Extend the reward tube before positioning the mouse on the ball to prevent any potential injury resulting from inadvertently extending the tube too far forward once the mouse is head fixed. Elevate the reward tube 5-15 mm above the spherical treadmill such that licking the spout requires a natural forward-facing posture of the head.
- Head fixing: To head-fix the mouse, place the mouse on the handler's dominant side of the spherical treadmill. Then, using the handler's dominant hand, pull the mouse by its headbar towards the head-fixing platform. Place the head-bar in the slot meant for fixing, after which using the handler's non-dominant hand, click the head-bar into place.
- Location on ball: Individualize placement on the spherical treadmill for each mouse but ensure they meet the following requirements to ensure motivation for tasting the reward and minimize overall levels of stress.
- Line the midsagittal plane of the mouse with the center of the spherical treadmill. In cases where the head-bar is not straight, ensure that the midsagittal plane of the mouse, rather than the head-bar, is in line with the center of the placement. For visual clarity, see Figure 3C.
- Ensure that the mouse's hind paws are no more than 11 cm from the apex of the spherical treadmill and that the head is behind the apex. Ensure that all four paws are touching the treadmill and that the abdomen can touch the treadmill when the mouse is at rest; this will support proper gait and stability on the ball for running.
- When mice do not run, this is called ball refusal. If the mice continue to freeze and do not attempt to run, they are likely experiencing excessive anxiety, and as the investigator choose to exclude them from the experiment. In this study, a quantitative threshold of 5 days of ball refusal was used to determine exclusion from the data.
- Side bias: When mice first start habituating to the training routine, they will favor one side over the other. This can interfere with task performance, so take care to ensure that any side preference is not due to asymmetry in how the animal is mounted on the ball. The y-maze task employed here specifically requires the animal to make both right and left choices to optimize reward delivery, which facilitates overcoming side preferences.
- Reward spout: This approach involves a gentle maneuver referred to as the kiss it method, where the mouse is guided towards the extended lick tube until its mouth almost touches the tip of the spout, thus ensuring accurate delivery of the reward. Set the duration of the extended reward tube to 1 s when mice receive rewards, permitting the mouse adequate time to consume the droplet fully. Individualize lick spout positioning for each mouse, as the size and preferred positioning for each individual mouse may differ. Ensure the reward tube stays centered throughout all trials for licking standardization; the mouse should always expect to receive the reward in the same physical location regardless of the virtual maze design.
NOTE: While the determination of this duration is at the discretion of the investigator, these findings indicate that this timeframe was effective in facilitating complete reward intake by the mouse before the retraction of the tube. Figure 3B displays an example of a preferable positioning for placement. - Linear track: Consistent with prior studies employing similar methodologies, use a linear track task to investigate two key inquiries: the time required to train mice to traverse a straight corridor and the anticipated success rate of reward acquisition by mice.
- Ensure mice have acclimated to both the fluid restriction paradigm and the experimental hardware.
- Perform a daily 30 min session to move along a linear virtual corridor starting with a 1 m length. Upon reaching the end of the corridor and receiving the sugar droplet reward, teleport the mice back to the starting point.
- Determine a criteria-based advancement to longer mazes (e.g., 1 m, 2 m, 3 m). Advance the mice to the next maze length after achieving 2 consecutive days of receiving an average of 2 rewards per min (Figure 4A).
- Document daily recordings of timestamped data concerning reward retrieval, and the distance covered by mice on the spherical treadmill for further analysis (Figure 4B-D).
- For mice receiving an average of 2 rewards per min on the 3 m linear tracks, mark them as proficient on the linear track paradigm. It is recommended that mice reach this phase before progressing to more complex behavioral tasks requiring decision-making.
- Complex Behavioral Tasks Requiring Decision-Making (Y-Maze): This phase explores the feasibility of progressing from a simple to a more complex behavioral task that requires decision-making. To accomplish this, create a devised signal discrimination Y-Maze task.
- In this Y-maze paradigm23,24, ensure the mice navigate towards a choice point where two arms extend out 45° in either direction like the shape of a Y. Deactivate rotation from the starting point of the maze until reaching the choice point, two arms of varying color, and then activate it within the decision zone to allow the mouse to pivot towards its desired direction.
- Upon entry into the arm leading to the reward zone, deactivate the rotation once again. A black arm represents the correct path, while a white arm represents the incorrect path. Use the black arm and white arm as cues to accommodate for potential limitations in mouse visual acuity as they are easily distinguishable, facilitating an examination of their usage of visual information in its simplest form.
- Train the mice to navigate towards the black arm to obtain a sugar reward, with each trial concluding with the mice being teleported back to the starting location. Incorporate in the experimental design a random shuffling of the reward location between the left and right sides, ensuring that mice associate the reward with the visual cues rather than the specific side.
- Use the same steps for setting up the Y-maze as the linear corridor. Mirror the criteria for progression in the Y-maze paradigm to those of the linear corridor: each trial lasts 30 min, and mice need to reach a predetermined reward threshold for 2 consecutive days. A threshold of 70% rewards correctly acquired is recommended based on the average performance of previous pilot cohorts in the Y-maze; it is above the chance threshold (50%), and it represents a reasonably attainable percentage indicative of mice comprehending the task (Figure 5A).
- Upon reaching the choice point, ensure the mouse selects one of the correct or incorrect arms. At the end of the arm, teleport it back to the starting point to repeat the maze within a 30 min timeframe.
- This approach employed a visual psychophysics-inspired approach where the mazes got progressively more challenging to distinguish. Follow the description below for the progression in the Y-maze paradigm.
- In the initial Y-maze, present solid black and white arms at the maze's choice point. If the mouse correctly chose the black arm for 70% of the trials for 2 consecutive days, progress it to a subsequent level with increasingly challenging discrimination tasks. To achieve this, gradually introduce an additional 10% of the contrasting color to each arm at every level of progression. For example, transition the white arm to being composed of 90% white and 10% black, and vice versa, rendering discrimination more demanding with each advancement.
NOTE: The idea for increasing is if a 50% white/black can be reached, it would be an effective control as the arms would be indistinguishable. However, the furthest the mice were able to visually discriminate against was 80%:20% (Figure 5B).
- In the initial Y-maze, present solid black and white arms at the maze's choice point. If the mouse correctly chose the black arm for 70% of the trials for 2 consecutive days, progress it to a subsequent level with increasingly challenging discrimination tasks. To achieve this, gradually introduce an additional 10% of the contrasting color to each arm at every level of progression. For example, transition the white arm to being composed of 90% white and 10% black, and vice versa, rendering discrimination more demanding with each advancement.
Representative Results
This pilot study aimed to outline methodologies for the efficient training of mice in two distinct tasks: a simple corridor and a complex decision-making task (the Y-maze visual discrimination task). These data served as the basis for establishing temporal guidelines for behavioral training in VR.
The procedural steps start by outlining the surgical implantation of the head-bar in Figure 1. This implant serves to stabilize the mouse's skull during behavioral assessments, thereby enhancing the precision of neural recordings, particularly when employed in conjunction with electrophysiology or imaging techniques.
Figure 2 and Figure 3 illustrate the hardware components and setup of the experimental system. Figure 2 details the water delivery system, which utilized a Petri dish fountain method. This involved affixing a 60 mm x 15 mm Petri dish concave-side down onto the cage floor, securing a smaller 35 mm x 10 mm Petri dish concave-side down at the center of the larger dish, and placing another 60 mm x 15 mm Petri dish concave-side up on top of the smaller dish to serve as a water reservoir. The height of the upper dish was carefully adjusted to prevent contamination by bedding material while ensuring mice had easy access to water.
Figure 3 presents the system hardware and mouse positioning guidelines. Figure 3A depicts the VR setup, which featured a six-screen array with a spherical treadmill positioned centrally. Figure 3B shows the optimal placement of the mouse on the treadmill, with the head aligned in a natural position and all four paws in contact with the surface. Figure 3C compares correct and incorrect mouse placement relative to the head-bar, emphasizing that the midsagittal plane of the mouse should be centered, rather than aligning with the head-bar itself.
Figure 4 presents reward acquisition curves on a line graph, illustrating the expected learning periods for 1 m, 2 m, and 3 m narrow corridors in VR based on predefined parameters for progression. It depicts the average velocities of mice across respective track lengths, demonstrating a gradual increase in speed as evidence of task learning and improvement commensurate with increased difficulty. A bar graph is also shown illustrating the average number of days required for mice to reach the criterion for the linear tracks, as well as a bar graph displaying mean velocities for each track length. Following this, the progressive stages of the linear track task learned by the mice are illustrated as well. These tasks were designed to replicate methodologies established in academic literature while ensuring a learning curve feasible for mice, facilitating their advancement through the levels.
Finally, Figure 5 provides data pertaining to the Y-Maze task. The figure illustrates the progressive nature of the task, beginning with a straightforward discrimination between solid black and white arms. This initial stage serves as a foundational step, establishing the mice's ability to distinguish between contrasting visual cues. Subsequent levels of the task introduce increasing complexity by incorporating additional percentages of the contrasting color to each arm, thereby challenging the mice's discrimination abilities further. The gradual augmentation of task difficulty is exemplified by the transition from solid black and white arms to arms composed of 90% of one color and 10% of the other. Notably, the data presented in Figure 5 indicates that while discrimination accuracy improves with each level of progression, some mice consistently demonstrate a threshold of visual discrimination capability, reaching a maximum of 80%/20% white/black discrimination. This observation underscores the limitations inherent in the mice's visual discrimination abilities within the context of the Y-Maze task, providing valuable insights into the task's feasibility and the cognitive capacities of the subjects. Subsequently, the progressive stages of the Y-maze track task, which were designed to align with established methodologies in the literature, are detailed. These stages ensured a feasible learning curve for the mice, supporting their gradual advancement through the levels.
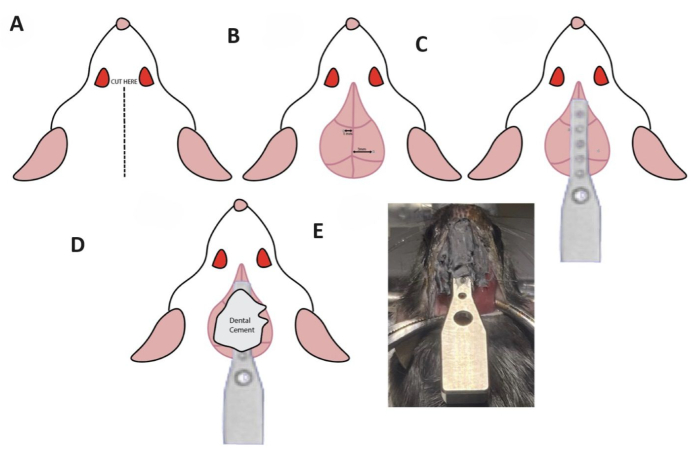
Figure 1: Surgical instructions for head-bar implantation. (A) The incision site is marked on the mouse's cranium. (B) The screws should be implanted 1 mm to the left of the interfrontal suture slightly below bregma and 3 mm to the right of the interfrontal suture slightly above lambda. (C) The head-bar should be placed along the interfrontal suture. (D) Apply dental cement over the head-bar implant. (E) Actual visualization of the head-bar after the application of dental cement. Please click here to view a larger version of this figure.
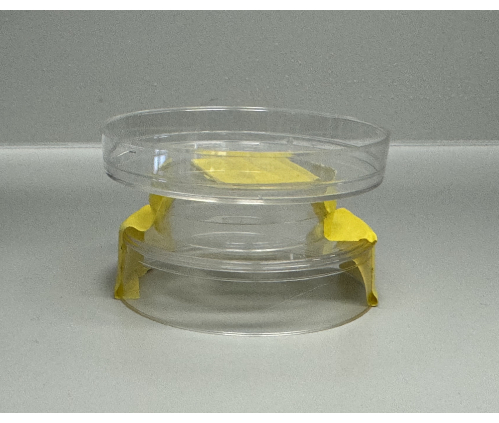
Figure 2: Water delivery system using a petri dish fountain method. A 60 mm x 15 mm Petri dish was fixed concave-side down on the cage floor. A smaller 35 mm x 10 mm petri dish was centered on the larger dish, with another 60 mm x 15 mm Petri dish placed concave-side up on top to serve as a reservoir. This setup ensured that the water remained uncontaminated by bedding and accessible to the mice. Please click here to view a larger version of this figure.
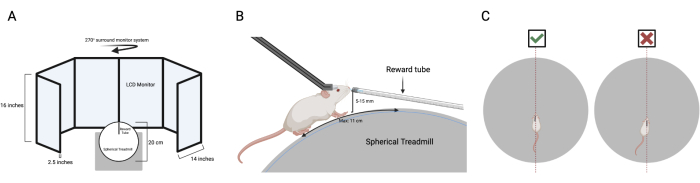
Figure 3: System hardware and positioning of the mouse guidelines. (A) This displays the VR setup utilized. A six-screen setup was utilized, with the spherical treadmill placed in the middle. (B) Side view of optimal mouse placement on the spherical treadmill. The mouse head is in a natural position, while all four paws are on the spherical treadmill. (C) Top view of correct versus incorrect placement of the mouse in regard to the head-bar. For correct placement, the midsagittal plane of the mouse should be centered rather than the head-bar itself. Please click here to view a larger version of this figure.
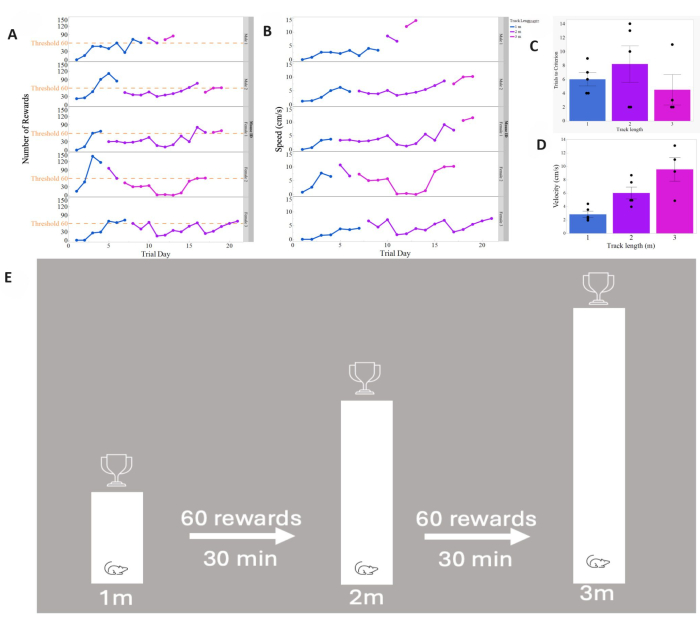
Figure 4: Linear track data. (A) The presented data depict the daily rewards collected within each 30 min trial period. Mice progressed to longer track lengths once they achieved an average of 2 rewards per min over 2 consecutive days, totaling 60 rewards (threshold). (B) As mice acquired proficiency in the task, their velocities exhibited a gradual increase, indicative of the efficacy of reward reinforcement. The graph illustrates the average daily velocity of each mouse on the track in cm/s, portraying a linear progression in learned behavior. (C) This bar graph illustrates the duration taken by each mouse to acquire proficiency on individual track lengths, with the respective means and standard error depicted for each track length. (D) This bar graph demonstrates the mean and standard error of the average daily velocities achieved by each mouse across various track lengths. The nearly linear progression suggests a learned enhancement in running speed. (E) This illustrates the progression of the linear track task, which requires 2 consecutive trial days of 60 rewards before advancement to a longer version of the maze. Please click here to view a larger version of this figure.

Figure 5: Y-Maze data. (A) This shows the distribution of rewards acquired at different stages of the Y-maze progression. This analysis focused exclusively on a subset of four mice that completed all phases of the linear track, thereby ensuring an equitable representation of both male and female participants. (B) This visual representation illustrates the stages of the Y-Maze task, wherein mice advance upon achieving two consecutive days of 70% correct choices. Please click here to view a larger version of this figure.
Discussion
This study employed a comprehensive approach to investigate the behavioral responses of mice in VR environments, focusing on the implementation of surgical procedures, fluid restriction protocols, system setup, and behavioral tasks. These findings contribute to the field by providing procedural details, time frames for training, and success rates. This will enable more effective adoption of VR procedures in mice and facilitate planning and implementation for labs interested in using this procedure in their research.
The surgical implantation of head-bars was essential for facilitating head-fixed behavioral experiments in VR environments. By carefully following established protocols and providing appropriate post-operative care, the successful integration of head-bars was ensured while minimizing adverse effects on the animals’ health and behavior. Additionally, fluid restriction protocols were implemented to regulate water intake and maintain hydration and thirst levels among the mice. The gradual acclimation process and periodic access to water were crucial for ensuring the animals’ welfare while facilitating the execution of behavioral tasks.
The setup of the VR behavioral system involved the integration of hardware and software components to create immersive virtual environments for the mice. The utilization of fully immersive virtual displays, liquid reward systems, styrofoam balls as spherical treadmills, and head holders enabled precise control over experimental conditions and data acquisition. Behavioral tasks, including the linear track and Y-maze paradigms, were carefully designed to investigate key aspects of mouse behavior, such as locomotion, decision-making, and reward processing.
Despite best efforts to optimize experimental procedures, several challenges were encountered during the study. Variability in individual mouse responses and technical issues related to hardware and software integration posed challenges to data collection and analysis. Additionally, the reliance on fluid restriction protocols necessitated careful monitoring of the animal’s hydration status and adjustment of experimental procedures accordingly. At times, mice would struggle when placed on the ball, not drink from the reward spout, or freeze and fail to run on the ball. Although some of these challenges may be temporary, it is crucial to monitor the mice to ensure they are not experiencing impediments in their progress. Mice that fail to show advancement compared to their peers should be withdrawn from the study. One similar experiment had 4 of 55 mice removed due to their inability to learn the paradigm25. Mice exhibiting consistent immobility on the ball for 5 consecutive days were excluded from the study following thorough assessments of their weight, ability to access the reward spout for drinking, and positioning on the ball to ensure no underlying issues were present. In these cases, it is up to the discretion of the researcher to decide what strategy to take to resume the study efficiently.
These training protocols were designed to progressively challenge the mice while ensuring their proficiency in executing behavioral tasks. Criteria for progression from the linear track to the Y-maze paradigm were based on the mice’s ability to meet predetermined performance thresholds, such as achieving consecutive days of successful trials and reward acquisition. The implementation of rigorous training protocols allowed us to assess the mice’s behavioral capabilities and adaptability to increasingly complex tasks. These carefully structured protocols provide a robust framework for researchers in the field of behavioral neuroscience, offering a systematic approach to evaluating and training animals for diverse experimental paradigms. By outlining clear criteria for progression, researchers can efficiently gauge the learning curve of experimental subjects and curate training paradigms accordingly. Furthermore, this methodological approach fosters reproducibility and standardization across experiments, facilitating comparative analyses and advancing the understanding of cognitive processes and learning mechanisms in animal models.
When designing a VR paradigm for mice, it is crucial to recognize the range of approaches available concerning task complexity and training progression. This protocol offers a broad framework for constructing an experimental design, yet it remains up to the investigator to tailor specific aspects such as reward delivery, bias control, stimulus type, task progression, and system parameters according to the needs of the study. For instance, some studies opt for a more streamlined approach, focusing on immediate task engagement. An example is Krumin et al. which implemented a single, consistent T-maze task rather than employing a progressive learning regimen between different tasks. In contrast, other studies offer diverse trial design components, such as stimulus reinforcement strategies and auditory cues. The study utilized auditory feedback as a punishment for incorrect trials and provided only water as a reward for correct trials26. Conversely, Zhao et al. employed a 10% sucrose solution as a reward for correct trials and did not incorporate any form of punishment for incorrect trials27. Instead, they focused on mitigating incorrect responses through methods such as anti-bias training, which involved increasing the probability of switching the cue direction from the animal’s previous choice and adjusting the daily water allowance to enhance motivation. Differences in experimental design, such as the presence of spatial cues throughout the task, can lead to varying interpretations of neural coding, as evidenced by Zhao et al. finding posterior parietal cortex cell selectivity explained by trajectories and spatial preferences, in contrast to Harvey et al.’s observed choice-dependent activation sequences27,28. It is important to note that the specific hardware used included six LCD monitors, an extendable lick spout, and an air-cushioned styrofoam ball treadmill. There are a number of differences across virtual reality systems across labs, including the use of projectors29 versus computer monitors, non-spherical treadmills30, and fixed10 versus extendable lick spouts.
In conclusion, this study provides valuable insights into the behavioral responses of mice in VR environments and demonstrates the feasibility of employing immersive technology to investigate complex behaviors. Future research endeavors may focus on refining experimental protocols, exploring neural mechanisms underlying decision-making processes, and translating findings to clinical applications. By continuing to advance the understanding of mouse behavior, scientists can further elucidate the neural circuits and cognitive processes underlying complex behaviors in both health and disease.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This research was funded by the National Institutes of Environmental Health Sciences (ZIC-ES103330). Special thanks to K. Krepinksy of Phenosys for his help on the hardware and software properties of the system, to T. Viney of the University of Oxford for his assistance with behavioral paradigms, and finally to G. Vargish of the NIH for his guidance on his piloting procedures and surgical methods.
Materials
| 2.4 mm Screws (00-96 X 3/32) | Protech International | 8L0X3905202F | For Added Headbar Stability |
| Bupivocaine | Hospira | NDC:0409-1162-19 | Local Anesthetic |
| Buprenorphine | Wedgewood Pharmaceuticals | SKU: BUPREN-INJ010VC | Analgesia |
| Buzzers | Wahl | 1565q | For Shaving Surgical Region |
| Drill and microinjection robot | Neurostar | 17129-IDA | Stereotaxis |
| GLUture | Zoetis | 32046 | Surgical Adhesive |
| Head-bar Implant | Luigs-Neumann | 130060 | Mouse Head Implant |
| Heating Pad (Lectro-Kennel) | K&H Manufacturing | 100212933 | Post-operative |
| Hemostats | World Precision Instruments | 501291 | Surgical Tool |
| Hydrogen Peroxide | Swam | L0003648FB | Cleaning Agent |
| Isoflurane | Dechra | B230008 | Surgical Inhalation Anesthetic |
| Isoflurane/O2 Delivery device w Nosecomb attachments | Eagle Eye Anesthesia Inc. | Model 50 Anesthesia | Surgical Device |
| Metabond | Parkell | CB-S380 | Adhesive Cement |
| Microscissors | Fine Science Tools | 15000-08 | Surgical Tool |
| Oxygen | Praxair | UN1072 | Surgical Oxygen |
| Povidone-Iodine Swabstick | Dynarex | g172095-05 | Surgical Tool |
| Saline | Hospira | NDC:0409-1966-02 | Hydration Agent |
| Sterile Cotton Tipped Applicator (Q-tips) | Puritan | 25-806 2WC | Surgical Tool |
| Sucrose | Fisher Chemical | CAS 57-50-1 | Primary Reinforcer/Motivator/Reward |
| Tweezers | World Precision Instruments | 504505 | Surgical Tool |
| Virtual Reality System | PhenoSys | JetBall-TFT | The JetBall, an air cushioned spherical treadmill allows an animal to navigate effortlessly in a virtual world projected on 6 surrounding monitors. |
| White petrolatum lubricant eye ointment ointment | AACE Pharmaceuticals | NDC:71406-124-35 | Eyelube |
Referenzen
- Guo, Z. V., et al. Procedures for behavioral experiments in head-fixed mice. PLoS One. 9 (2), e88678 (2014).
- Yang, Y., Kim, G. Headpost surgery for in vivo electrophysiological recording in the mouse inferior colliculus during locomotion. Bio Protoc. 10 (23), e3840 (2020).
- Fuhrmann, F., et al. Locomotion, Theta oscillations, and the speed-correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron. 86 (5), 1253-1264 (2015).
- Dombeck, D. A., Harvey, C. D., Tian, L., Looger, L. L., Tank, D. W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci. 13 (11), 1433-1440 (2010).
- Dombeck, D. A., Khabbaz, A. N., Collman, F., Adelman, T. L., Tank, D. W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 56 (1), 43-57 (2007).
- Leinweber, M., et al. Two-photon calcium imaging in mice navigating a virtual reality environment. J Vis Exp. (84), e50885 (2014).
- Chen, X., et al. Sensory evoked fMRI paradigms in awake mice. Neuroimage. 204, 116242 (2020).
- Burgess, C. P., et al. High-yield methods for accurate two-alternative visual psychophysics in head-fixed mice. Cell Rep. 20 (10), 2513-2524 (2017).
- Giovannucci, A., et al. Automated gesture tracking in head-fixed mice. J Neurosci Methods. 300, 184-195 (2018).
- Aghajan, Z. M., et al. Impaired spatial selectivity and intact phase precession in two-dimensional virtual reality. Nat Neurosci. 18 (1), 121-128 (2015).
- Cushman, J. D., et al. Multisensory control of multimodal behavior: do the legs know what the tongue is doing. PLoS One. 8 (11), e80465 (2013).
- Thurley, K., Ayaz, A. Virtual reality systems for rodents. Curr Zool. 63 (1), 109-119 (2017).
- Forro, T., Klausberger, T. Differential behavior-related activity of distinct hippocampal interneuron types during odor-associated spatial navigation. Neuron. 111 (15), 2399-2413.e5 (2023).
- Cho, W. H., et al. Hippocampal astrocytes modulate anxiety-like behavior. Nat Commun. 13 (1), 6536 (2022).
- Lee, B. H., et al. Real-time visualization of mRNA synthesis during memory formation in live mice. Proc Natl Acad Sci U S A. 119 (27), e2117076119 (2022).
- Leinonen, H., Tanila, H. Vision in laboratory rodents-Tools to measure it and implications for behavioral research. Behav Brain Res. 352, 172-182 (2018).
- Whishaw, I. Q. A comparison of rats and mice in a swimming pool place task and matching to place task: some surprising differences. Physiol Behav. 58 (4), 687-693 (1995).
- Pinto, L., et al. An accumulation-of-evidence task using visual pulses for mice navigating in virtual reality. Front Behav Neurosci. 12, 36 (2018).
- Tirado-Muniz, N., et al. Evaluation of cage mate-induced postsurgical trauma in mice. J Am Assoc Lab Anim Sci. 62 (2), 170-178 (2023).
- Barkus, C., et al. Refinements to rodent head fixation and fluid/food control for neuroscience. J Neurosci Methods. 381, 109705 (2022).
- Harvey, C. D., Collman, F., Dombeck, D. A., Tank, D. W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 461 (7266), 941-946 (2009).
- Peirson, S. N., Brown, L. A., Pothecary, C. A., Benson, L. A., Fisk, A. S. Light and the laboratory mouse. J Neurosci Methods. 300, 26-36 (2018).
- Wenk, G. L. Assessment of spatial memory using the T maze. Curr Protoc Neurosci. Chapter 8, Unit 8 5B (2001).
- d’Isa, R., Comi, G., Leocani, L. Apparatus design and behavioural testing protocol for the evaluation of spatial working memory in mice through the spontaneous alternation T-maze. Sci Rep. 11 (1), 21177 (2021).
- Viney, T. J., et al. Spread of pathological human Tau from neurons to oligodendrocytes and loss of high-firing pyramidal neurons in aging mice. Cell Rep. 41 (7), 111646 (2022).
- Krumin, M., Lee, J. J., Harris, K. D., Carandini, M. Decision and navigation in mouse parietal cortex. Elife. 7, e42583 (2018).
- Zhao, X., Hsu, C. L., Spruston, N. Rapid synaptic plasticity contributes to a learned conjunctive code of position and choice-related information in the hippocampus. Neuron. 110 (1), 96-108.e4 (2022).
- Harvey, C. D., Coen, P., Tank, D. W. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 484 (7392), 62-68 (2012).
- Pettit, N. L., Yap, E. L., Greenberg, M. E., Harvey, C. D. Fos ensembles encode and shape stable spatial maps in the hippocampus. Nature. 609 (7926), 327-334 (2022).
- Pinke, D., Issa, J. B., Dara, G. A., Dobos, G., Dombeck, D. A. Full field-of-view virtual reality goggles for mice. Neuron. 111 (24), 3941-3952.e6 (2023).

.
