Investigating Scarless Tissue Regeneration in Embryonic Wounded Chick Corneas
Summary
The present protocol demonstrates the different steps involved in wounding the cornea of an embryonic chick in ovo. The regenerating or fully restored corneas can be analyzed for regenerative potential using various cellular and molecular techniques following the wounding procedure.
Abstract
Chick embryonic corneal wounds display a remarkable capacity to fully and rapidly regenerate, whereas adult wounded corneas experience a loss of transparency due to fibrotic scarring. The tissue integrity of injured embryonic corneas is intrinsically restored with no detectable scar formation. Given its accessibility and ease of manipulation, the chick embryo is an ideal model for studying scarless corneal wound repair. This protocol demonstrates the different steps involved in wounding the cornea of an embryonic chick in ovo. First, eggs are windowed at early embryonic ages to access the eye. Second, a series of in ovo physical manipulations to the extraembryonic membranes are conducted to ensure access to the eye is maintained through later stages of development, corresponding to when the three cellular layers of the cornea are formed. Third, linear cornea wounds that penetrate the outer epithelial layer and the anterior stroma are made using a microsurgical knife. The regeneration process or fully restored corneas can be analyzed for regenerative potential using various cellular and molecular techniques following the wounding procedure. Studies to date using this model have revealed that wounded embryonic corneas display activation of keratocyte differentiation, undergo coordinated remodeling of ECM proteins to their native three-dimensional macrostructure, and become adequately re-innervated by corneal sensory nerves. In the future, the potential impact of endogenous or exogenous factors on the regenerative process could be analyzed in healing corneas by using developmental biology techniques, such as tissue grafting, electroporation, retroviral infection, or bead implantation. The current strategy identifies the embryonic chick as a crucial experimental paradigm for elucidating the molecular and cellular factors coordinating scarless corneal wound healing.
Introduction
The cornea is the transparent, outer-most tissue of the eye that transmits and refracts light conducive to visual acuity. In the adult cornea, damage or infection to the corneal stroma leads to a rapid and robust wound healing response characterized by keratocyte proliferation, fibrosis, increased inflammation leading to cytokine-induced apoptosis, generation of repair myofibroblasts, and overall remodeling of the extracellular matrix (ECM)1,2. Following injury, such corneal tissue repair results in opaque scar tissue that reduces corneal transparency and occludes the passage of light, thus distorting vision and, in the most severe cases, leading to corneal blindness3. Thus, there is a clear need to develop reliable animal models to address the complexities of wound healing and to identify the cellular and molecular factors responsible for wound closure and tissue regeneration.
To date, most studies examining corneal wound healing have utilized post-natal4 or adult animal models1,2,5,6,7. While these studies have led to a significant advancement in the understanding of the corneal wound healing response and the mechanisms underlying scar formation, the damaged corneal tissues in these healing models fail to fully regenerate, thus limiting their utility for identifying the molecular factors and cellular mechanisms responsible for fully recapitulating corneal morphology and structure post-injury. By contrast, fetal wounds generated with a knife in the embryonic chick cornea possess an intrinsic capacity to heal fully in a scarless fashion8. Specifically, the embryonic chick cornea exhibits nonfibrotic regeneration with the complete recapitulation of the extracellular matrix structure and innervation patterns8,9.
The present protocol describes a sequence of steps involved in wounding the cornea of an embryonic chick in ovo. First, eggs are windowed at early embryonic ages to facilitate access to the embryo. Second, a series of in ovo physical manipulations to the extraembryonic membranes are conducted to ensure access to the eye is maintained through later stages of development, corresponding to when the three cellular layers of the cornea are formed and wounding is desired. Third, linear central cornea incisions penetrating through the corneal epithelium and into the anterior stroma are made using a microsurgical knife. The regeneration process or fully restored corneas can be analyzed for regenerative potential using various cellular and molecular techniques following the wounding procedure.
Protocol
The strain of eggs used in this protocol was White Leghorn, and all animal procedures were approved by the Institutional Animal Care and Use Committee at Illinois Wesleyan University.
1. Incubation of chick eggs
- Keep the eggs at ~10 °C for up to 1 week after they are laid to halt development. When ready to initiate chick embryo development, wipe the entire eggshell with lint-free wipes (see Table of Materials) saturated with room temperature water to remove dirt and debris.
- Ensure the eggshell is sanitized. Wipe down the entire egg surface with lint-free wipes dampened with 70% ethanol. Quickly wipe off the ethanol to dry the egg and avoid ethanol absorption through the eggshell to the embryo.
- Arrange the eggs horizontally on a tray. Mark the top of the egg to denote the expected position of the embryo. Incubate the eggs horizontally, with the rocking function activated, in a 38 °C humidified incubator.
2. Windowing the eggs to prepare for membrane dissection
- Remove eggs from the incubator on the third day of embryonic development (E3). Sterilize the top of the eggs with lint-free wipes dampened with 70% ethanol. Dry the ethanol from the eggshell surfaces.
NOTE: To ensure that development was not delayed during the windowing procedure, 6-12 eggs were removed, and the procedure was quickly carried out on these while the remaining eggs were left in the incubator. - Position an egg horizontally in a secure egg holder (see Table of Materials). Using the sharp end of dissecting scissors, create a small hole in the top of the eggshell near the pointed end of the egg.
NOTE: This hole will facilitate the removal of albumen, which is necessary to drop the yolk and embryo away from the inner eggshell surface. For egg holders, paper-pulp egg filler flats, within which the eggs come shipped, were used. - Through the hole (Step 2.2.), insert an 18 G beveled hypodermic needle. With the needle pushed to the bottom inner surface of the egg and the bevel-side of the needle facing the pointed end of the egg (e.g., away from the expected location of the yolk and the embryo near the middle of the egg), remove 2-3 mL of albumen from the chicken egg and discard.
NOTE: If one's needle nicks the embryo or its associated vasculature during this step, it will result in blood being aspirated with the albumen. This will result in embryo death. Further, if the yolk is inadvertently aspirated along with the albumen during this step, the embryo will not be viable. In either case, the egg should be discarded if the embryo cannot be immediately used for other purposes. - Clean the eggshell surface surrounding the hole with lint-free wipes lightly dampened with 70% ethanol and wipe dry. Seal the hole made for removing albumen with clear tape.
- With the sharp end of dissecting scissors, make a second "window" hole in the top of the eggshell at the marking site (Step 1.3.). Ensure that the scissors do not extend too far into the eggshell to avoid contacting and damaging the embryo or embryonic vasculature, which will often be positioned within the egg directly below the site of the second hole.
- Using curved iris forceps, widen the "window" hole to span ~2-3 cm in diameter and serve as a "window" to the developing embryo beneath the shell.
- Insert one end of the forceps into the hole, keeping it parallel to and closely juxtaposed with the eggshell. With the other forceps end positioned outside the eggshell, carefully pinch the two forceps ends together, allowing them to break and remove small pieces of the eggshell. Continue breaking and removing eggshell fragments until there remains a 2-3 cm window that directly overlays the embryo.
NOTE: Eggs in which embryos do not lay directly beneath the hole made in Step 2.6. must not be used as the forthcoming membrane dissections would be challenging to complete. Even with rocking the eggs horizontally, about 10% of the eggs are unusable due to the poor positioning of the embryo. These embryos can be used for other purposes.
- Insert one end of the forceps into the hole, keeping it parallel to and closely juxtaposed with the eggshell. With the other forceps end positioned outside the eggshell, carefully pinch the two forceps ends together, allowing them to break and remove small pieces of the eggshell. Continue breaking and removing eggshell fragments until there remains a 2-3 cm window that directly overlays the embryo.
- To limit bacterial contamination, add through the window hole (e.g., into the egg) ~100-200 µL of Ringer's solution (8 g of NaCl, 0.37 g of KCl, and 0.23 g of CaCl2.2H20 per L of distilled H20) containing Penicillin/Streptomycin antibiotics (50 U/mL of Penicillin and 50 µg/mL of Streptomycin, see Table of Materials).
- Seal the window hole using clear adhesive tape. Perform the egg sealing by aligning a corner of the tape on the long axis of the hole and pressing the tape to the shell ~1-2 cm away from the edge of the hole.
- Continue sealing around the opening until a hanging flap of tape is left on one side. Press the two pieces of tape together, creating a domed shape over the hole, and press the flap of over-hanging tape to the shell to finish sealing the egg.
NOTE: Eggs need to be windowed at either E2 or E3. As per experience, windowing prior to E2 results in low embryo viability. Moreover, by E4, the embryo and extraembryonic membranes become attached to the eggshell10, and any attempts to window at E4 or later often result in embryo damage or tearing of extraembryonic blood vessels, with either event leading to the outcome of embryo death.
- Continue sealing around the opening until a hanging flap of tape is left on one side. Press the two pieces of tape together, creating a domed shape over the hole, and press the flap of over-hanging tape to the shell to finish sealing the egg.
- Return the "windowed" eggs to the incubator for further development. Ensure to keep the eggs horizontal and turn off the incubator's rocking function.
- Repeat Steps 2.2.-2.9. for each egg.
3. Microdissections of the extraembryonic membranes
- Remove an E5.5 windowed egg from the incubator. Expose the embryo by cutting the tape away from the window with sterilized dissecting scissors.
- Use a dissecting microscope to observe the embryo and its extraembryonic membranes through the window. If necessary, use scissors or curved iris forceps to widen the window so that the embryo is well-positioned beneath the window, taking care not to damage the embryonic vasculature.
- Add two drops of Ringer's solution containing Penicillin/Streptomycin antibiotics to hydrate the embryo and sterilize the egg.
- Use a dissecting microscope to ensure the embryo is at the proper developmental stage (Hamburger Hamilton stage 27, ~E5.5)11,12 and locate the positions of the amniochorionic membrane (ACM) and the chorioallantoic membrane (CAM).
NOTE: At this stage, the embryo is surrounded by the ACM, which comprises the amniotic membrane and overlying chorionic membrane fused and partially covered by the highly-vascularized allantois, which extends from the embryo's gut region and fuses with the overlaying chorion to form the CAM11,12. The ACM is not heavily vascularized, which enables one to dissect these membranes to expose the embryo without damaging blood vessels and harming the embryo. - Perform extraembryonic membrane dissections at this stage (E5.5).
NOTE: E5.5 is the ideal time to carry out the extraembryonic membrane dissections. Dissecting the membranes earlier (e.g., at E4) before CAM formation reduces embryo accessibility at later stages11. Moreover, at E5.5, the embryo is only partially covered by the highly-vascularized CAM, yet over the next 1-2 days, the CAM quickly envelops and precludes further access to the embryo11,12. For this reason, membrane dissection at E6 or later is challenging as the risk of tearing blood vessels increases.- Use a pair of sterilized fine forceps to gently grasp the ACM and pull it away from the embryo. Then use sterilized micro-dissecting scissors to cut a hole in the ACM directly above the forelimb that extends from the membrane overlying the forelimb to the membrane overlying the head.
NOTE: This step relaxes the chorion and amnion membranes, thus making them easier to grab and further dissect with forceps in the next steps. See Figure 1 for a helpful schematic of how membranes are dissected through the eggshell window.
- Use a pair of sterilized fine forceps to gently grasp the ACM and pull it away from the embryo. Then use sterilized micro-dissecting scissors to cut a hole in the ACM directly above the forelimb that extends from the membrane overlying the forelimb to the membrane overlying the head.
- Use two pairs of fine, sterile forceps to gently grab the amnion in two adjacent positions between the ACM and the CAM (e.g., an area between the cut made above the forelimb and the nearest edge of the CAM).
- Carefully move each pair of forceps, both firmly gripping the amniotic membrane, away from one another, with one pair moving dorsally to the embryo and the other ventrally.
NOTE: This motion serves to tear the amnion further while also separating the ACM (which gets pulled in the dorsal direction with respect to the embryo by one pair of forceps) and the CAM (which gets pulled in the ventral direction with respect to the embryo by the other pair of forceps). - Ensure that the membranes are separated when the CAM no longer covers the embryo and the allantoic artery and vein, which emanate from the embryonic gut to the CAM, are readily apparent.
- Carefully move each pair of forceps, both firmly gripping the amniotic membrane, away from one another, with one pair moving dorsally to the embryo and the other ventrally.
- Use sterilized fine forceps to dissect and remove any remaining amnion membrane covering the embryo. It is most commonly observed that the remaining amnion will partially cover the caudal half of the embryo.
- Using sterilized forceps, grasp the amnion near the mid-cranial region of the embryo and carefully pull the amnion in a caudal direction with respect to the embryo toward the earlier displaced CAM. The embryo will now be fully exposed, and further growth of the CAM will mainly occur away from the developing embryo.
NOTE: See Figure 1 for a helpful schematic on how the exposed embryo will appear following membrane dissection. Also, refer to a previously published report11 for helpful schematic diagrams of Steps 3.3.-3.6.
- Using sterilized forceps, grasp the amnion near the mid-cranial region of the embryo and carefully pull the amnion in a caudal direction with respect to the embryo toward the earlier displaced CAM. The embryo will now be fully exposed, and further growth of the CAM will mainly occur away from the developing embryo.
- Add a few drops of Ringer's solution containing Penicillin/Streptomycin antibiotics to hydrate the embryo and sterilize the egg.
- Reseal the window hole using clear tape, as described in Step 2.8. Return the egg to the incubator for further development, keeping the egg horizontal and keeping the incubator's rocking function inactivated.
- Repeat Steps 3.1.-3.8. for each egg.
NOTE: The extraembryonic membrane dissections at E5.5 described above will enable access to the embryo through E7, which is when wounding can be carried out8,9. By E8, the continued growth of the CAM tissue begins to cover the cranial region of the embryo, thus precluding further access to the cornea. If one desires to carry out wounding in older E8-E9 corneas, it is possible to reposition the growing CAM ventrally with respect to the embryo (Step 3.10.). If one wishes to wound at E7, Step 3.10. is not necessary to perform and one may proceed to Step 4, corneal wounding. - Remove an E7 egg from the incubator whose extraembryonic membranes were previously dissected at E5.5 (Steps 3.1.-3.8.). Grasp with sterilized forceps any available amnion membrane tissues fused to the CAM and gently pull the amnion membrane away from the cranial region of the embryo in a ventral direction with respect to the embryo.
NOTE: Since the amnionic membrane being displaced away from the embryo is fused to the CAM, the growing and highly vascularized CAM will follow the amnion-grasping forceps and move away from the cranial region. Repeat this step daily to continually displace the CAM away from the embryo until the embryo is at the desired age for wounding.
4. Corneal wounding
- Obtain an egg from the incubator for wounding at the desired embryonic age, E7-E9. Expose the embryo by cutting the tape away from the window with sterilized dissecting scissors. Add a few drops of Ringer's solution containing Penicillin/Streptomycin antibiotics to hydrate the embryo and sterilize the egg.
- Use a micro-dissecting knife to make an incision that spans the extent of the cornea of the right eye (due to how the embryo lays in the egg, the left eye is not accessible but can serve as a non-wounded control), which is parallel to and in line with the choroid fissure (Figure 1). The first cut will traverse the corneal epithelium.
- Use the micro-dissecting knife to again lacerate the cornea in the same spot as the first incision 2x more (e.g., three cuts total, with cut 2 and cut 3 occurring along with the same position in the cornea as cut 1)11. The second laceration will traverse the basement membrane, and the third will penetrate the anterior stroma.
NOTE: If the embryo has settled under the dissected CAM, one can use sterilized curved iris forceps to carefully move the head out from under the CAM. Position the curved iris forceps beneath the head, making contact with the left side of the head. Cradle the entire head on top of closed curved iris forceps and gently raise the head around and above the CAM. To help with viability, use a similar technique with the curved iris forceps to tuck the embryo back under the CAM after surgery to promote proper growth of the CAM.
- Use the micro-dissecting knife to again lacerate the cornea in the same spot as the first incision 2x more (e.g., three cuts total, with cut 2 and cut 3 occurring along with the same position in the cornea as cut 1)11. The second laceration will traverse the basement membrane, and the third will penetrate the anterior stroma.
- Add 3-4 drops of Ringer's solution containing Penicillin/Streptomycin antibiotics to hydrate the embryo and sterilize the egg.
- Reseal the window hole with clear tape and return to the incubator, leaving the egg horizontal. Allow the embryo to develop and the corneal wound to heal for a desired period (e.g., 0.5-11 days), and then humanely euthanize the embryo by decapitation.
- Use curved iris forceps to harvest the eye from a euthanized embryo floating in a Petri dish of Ringer's saline solution by gently grasping the eye on its posterior side, where the eye and facial tissue meet, and carefully lifting the whole eye away and free from the facial tissue.
- Use fine forceps to poke a small hole (3-5 cm) in the back of the whole eye and fix the whole eye in 4% paraformaldehyde at 4 °C overnight with mild agitation.
Representative Results
Following the earlier dissection of the ACM and CAM at E5.5 to expose the cranial region of the developing embryo, a series of lacerations that spanned the E7 central cornea was made in ovo (Figure 1). An ideal wound to study cornea regeneration occurs following three lacerations, each made in the same location of the cornea. The first laceration traverses the corneal epithelium, while the second and third lacerations penetrate the underlying basement membrane and anterior stroma, respectively. To achieve an ideal wound, it is crucial to use a sharp micro-dissection knife (see Table of Materials) and apply the correct amount of pressure as the laceration is made (Figure 2, see ideal wound). Applying too little pressure will result in a shallow wound that tears the corneal epithelium without sufficiently penetrating the anterior stroma (Figure 2, see shallow wound). Yet, applying too much pressure results in a full extent wound penetrating the entire stroma and exposing the aqueous humor to the external environment (Figure 2, see full extent wound).
Carrying out the proper lacerating incisions produces an ideal wound (Figure 2) that initially enlarges (0-3 days post wounding)8 (Figure 3). It has been postulated that the phase of wound enlargement that occurs by wounding E7 chicken corneas is related to the rapid expansion of eye size at this embryonic stage8. Embryonic chicken eyes grow at a significantly faster rate from E4 to E10 as compared to the eye growth from E10 to hatching. These early rapid phases of eye growth are attributed to elevated intraocular pressure (IOP)-dependent growth13. Therefore, it is likely that the rapid growth rate of the eye coupled with the elevated IOP promotes wound retraction during the early phases of the healing process (0-3 days post wounding), which is unique to the embryonic cornea wound healing progression. After that, re-epithelialization and new tissue formation occur (4-9 days post wounding) to ultimately close the wound in a scar-free fashion by 11 days post wounding8 (Figure 3A).
Further analysis of wound depth and regeneration was possible by staining cross-sections with a laminin antibody that marks the laminin-rich basement membrane and counter-staining the sections with the nuclear marker DAPI, which reveals the extent of the wound through the corneal epithelium8. Recently wounded corneas (0 dpw) and those that are early in the regeneration process (3 dpw) showed that the wound penetrated the epithelial layer and basement membrane, as evidenced by the break-in staining of the nuclear marker DAPI within the corneal epithelium and the absence of laminin antibody staining, which marks the laminin-rich basement membrane between the corneal epithelium and underlying stroma8 (Figure 3B). However, cross-sections through 11 dpw corneas stained with DAPI and laminin antibody revealed a completely healed cornea that had been re-epithelialized and contained a continuous laminin-rich basement membrane at the site of the regenerated wound8 (Figure 3B).
Following the corneal incision, detailed characterization of the wound healing process was accomplished by performing immunohistochemistry on sectioned, wounded corneal tissues. The extracellular matrix proteins fibronectin and tenascin are associated with epithelial and keratocyte cell migration into healing adult corneal wounds14,15. Spatiotemporal localization of the extracellular matrix proteins, fibronectin and tenascin is apparent within the healing wound and was found to be elevated at timepoints corresponding to corneal re-epithelialization (5 days post wounding)8 (Figure 4). Such analysis suggests the importance of fibronectin and tenascin to wound closure and, specifically, their involvement in epithelial cell migration and survival, consistent with such functions in adult corneal wounds16,17.
Commencing at E8-E9, the cornea becomes densely innervated by trigeminal sensory nerve fibers that emanate from a pericorneal nerve ring and traverse through the anterior stroma as they project toward the cornea's center and the corneal epithelium by E1218,19,20. Since corneal wounds in this model are made at E7, shortly before nerve projection into the cornea, this model further investigates corneal nerves as they navigate a healing cornea following insult. By using whole-mount immunohistochemistry to trace corneal nerves with anti-β neural tubulin (Tuj1) antibodies21, it is apparent that nerves are temporarily inhibited from the healing corneal tissue that directly juxtaposes the wounded, central cornea (5 days post wounding)8 (Figure 5A,B). Despite earlier inhibition, corneal nerves eventually innervate the fully healed corneal tissue (11 days post wounding) to similar density levels and in similar patterns to stage-matched, non-wounded controls (E18C) (Figure 5C,D).
Strikingly, fully re-epithelialized corneal tissues that have healed in a nonfibrotic, scarless fashion display a complete recapitulation of the normal collagen tissue architecture. As evidenced by second-generation harmonic imaging22,23, bundles of collagen fibers throughout varying depths of the central cornea wound area are arranged orthogonally, matching the native macrostructure of non-wounded central corneal tissue9 (Figure 6).
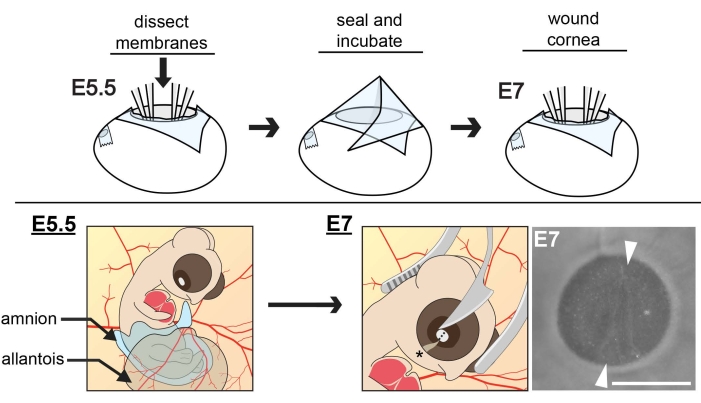
Figure 1: Schematic of in ovo extraembryonic membrane dissections and corneal wounding. At E5.5, the cranial region of the embryo is exposed by dissecting the ACM and CAM membranes and positioning the amnion and allantois away from the developing eye. Eggs are sealed and incubated to E7 when the central cornea is wounded, using curved forceps as a cradle for the embryo head as the tip of a microsurgical knife makes an incision in the central cornea. The wound is oriented parallel to the choroid fissure (asterisk). To ensure the depth of the incision reaches the anterior stroma, three concurrent cuts need to be made with the knife, each in the same relative position, one over the other. Scale bar = 1 mm. The figure is adapted with permission from references8,11. Please click here to view a larger version of this figure.
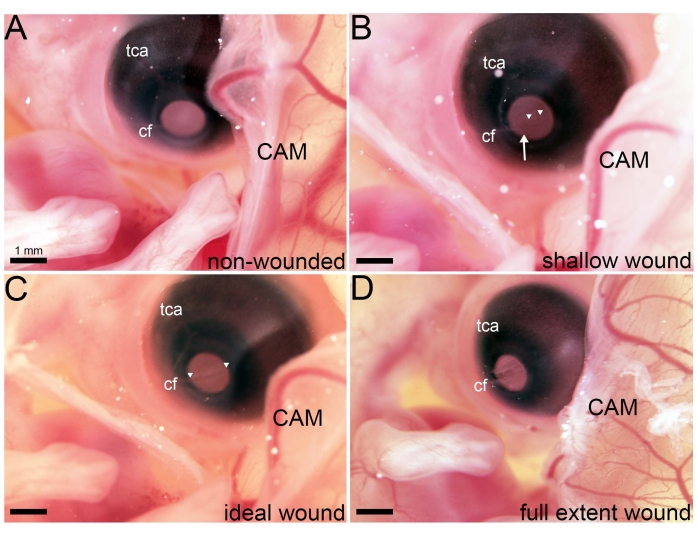
Figure 2: Variations in wounds generated in ovo. (A) Following membrane dissections at E5.5, the right eye is accessible in ovo. (B–D) Images taken of an in ovo embryo immediately following lacerations of varying degrees that span the cornea's extent and are in line with the choroid fissure (cf). (B) Following three lacerations wherein weak pressure was applied, a shallow wound is visible. The arrowhead marks a site in the cornea where the epithelium has been sheared but the anterior stroma has not been penetrated. Arrowheads denote a small cornea region where the anterior stroma has been penetrated. (C) Following three lacerations wherein an ideal amount of pressure was applied, an ideal wound is visible. Arrowheads denote a wound spanning the entire extent of the cornea where the anterior stroma has been penetrated. (D) Following three lacerations wherein excessive pressure was applied, a full extent wound is visible, and the aqueous humor has become exposed to the external environment. Abbreviations: tca, temporal ciliary artery; cf, choroid fissure; CAM, chorioallantoic membrane. Please click here to view a larger version of this figure.
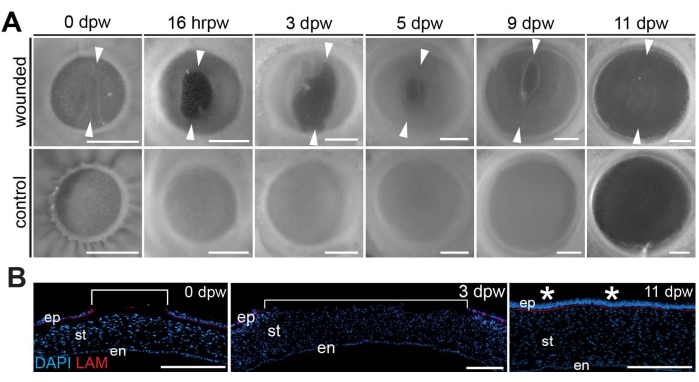
Figure 3: Wound healing progression. (A) Progression of healing in wounded corneas compared to stage-matched controls is shown from the time of wounding (0 dpw) and 16 h post-wounding (hrpw) through 3-11 days post wounding (dpw). Arrowheads delineate the dorsal- and ventral-most borders of the wound, indicating a period of wound expansion (0-3 dpw) followed by progressive wound closure (5-11 dpw). (B) Sectioned DAPI (blue)- and laminin (red)-stained wounded corneas at 0, 3, and 11 dpw. Brackets show the extent of the wounded region, which reveals wound expansion by 3 dpw and full repair of the re-epithelialized cornea by E11. Asterisks denote the healed region of the cornea. The scale bar is (A) 1 mm, (B) 100 µm. The figure is adapted with permission from reference8. Please click here to view a larger version of this figure.
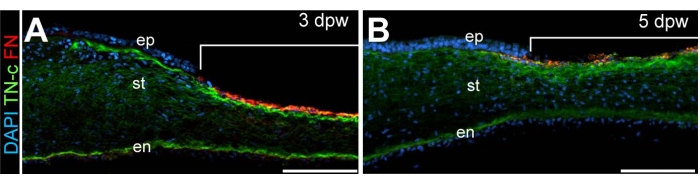
Figure 4: Histological analysis of wounded corneas. Cross-sections through (A) 3 dpw and (B) 5 dpw. DAPI-stained (blue) wounded corneas reveal the localization of fibronectin (FN, red) and Tenascin-C (TN-C, green). Brackets in (A) and (B) denote the wounded region. Scale bar: 100 µm. Abbreviations: ep, epithelium; st, stroma; en, endothelium. The figure is adapted with permission from reference8. Please click here to view a larger version of this figure.
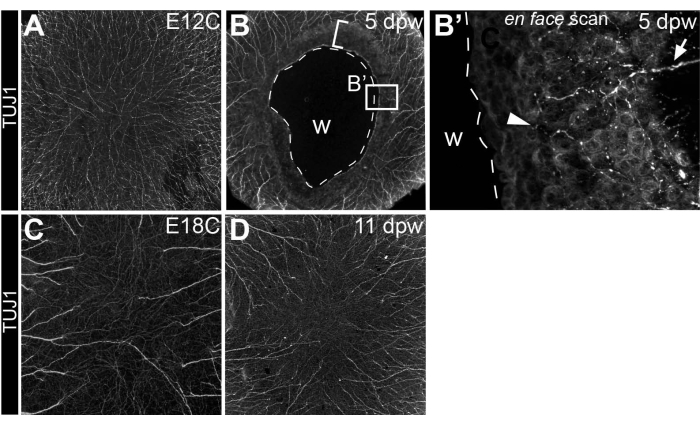
Figure 5: Innervation of wounded corneas. (A–D) Visualization of the corneal nerves following anti-β neural tubulin (Tuj1) whole-mount immunostaining in (B) 5 dpw and (D) 11 dpw corneas, as well as (A) stage-matched E12 (E12C, stage-matched for 5 dpw) and (C) E18 controls (E18C, stage-matched for 11 dpw). (B) The broken line in the 5 dpw cornea denotes the extent of the wound. The bracketed area directly adjacent to the wound denotes the cornea area that is temporarily repulsive to nerves and actively undergoing re-epithelialization. (B') Optical scan through the healing corneal tissue directly adjacent to the open wound reveals a rare stromal nerve bundle extending into the tissue (arrow) and epithelial nerve leashes (arrowhead). (C,D) Fully regenerated corneas at 11 dpw display similar innervation patterns and comparable nerve densities to stage-matched controls. Abbreviation: w, wound. The figure is adapted with permission from reference8. Please click here to view a larger version of this figure.
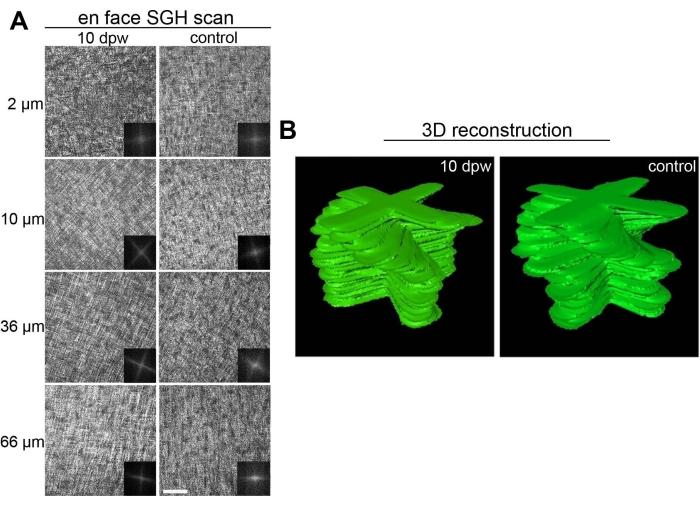
Figure 6: Collagen ultrastructure in healed embryonic corneas. (A) En face scan of fully healed 10 dpw corneas and stage-matched controls using second-generation harmonic imaging (SGH). Scan depths range from 2-66 µm from the anterior surface of the cornea (0 µm is the most anterior stroma) and are listed to the left of the respective images. Insets for each image correspond to Fast Fourier transform analysis of the central wound area for that particular scan depth. (B) Manually segmented stacks of two-dimensional Fast Fourier transform analysis, representing collagen organization within the wounded and stage-matched control cornea. Scale bar = 50 µm. The figure is adapted with permission from reference9. Please click here to view a larger version of this figure.
Discussion
The chick is an ideal model system for studying fetal, scarless cornea wound repair. Unlike mammals, the chick is easily accessible throughout development using in ovo8 or ex ovo strategies24. The embryonic chick cornea is much larger than rodent corneas, with nearly 50% of the cranial volume dedicated to the eye25, making it highly amenable to physical manipulations such as wounding. Moreover, chicken eggs are readily available year-round, often from local farms, and cost-effective, requiring only a humified incubator to support development.
This protocol reports a series of procedures that enable embryonic chick cornea wounding. Wounds made to the embryonic chick cornea fully regenerate, enabling a complete recapitulation of the native corneal structure with no detectable scarring. This technique has made the embryonic chick a vital animal model for elucidating the molecular and cellular factors coordinating scarless corneal wound healing.
Despite the clear promise inherent in the fetal wound healing model described herein, it is worth noting that there are clear differences between fetal and adult corneal wound healing. The embryonic cornea expresses growth factors and morphogenetic signals that are silenced or absent in adult tissues26. Moreover, wounded adult corneal tissues exhibit fibrosis and form scar tissue, likely due to a heightened inflammatory response mediated by cytokines and growth factors27, which are dampened or not yet established at embryonic stages. Such age-related differences within the corneal tissue could complicate efforts to restore adult wounded tissue fully. Nevertheless, determining key molecular factors and matrix proteins that regulate fetal scarless wound healing will pave the way for therapies that foster a more restorative healing process with less scarring and better recapitulation of the normal tissue architecture.
The wounding method described here builds upon a technique first developed by Spurlin et al.11 to gain in ovo access to late-stage chick embryos (e.g., >E6). By windowing the egg and dissecting extraembryonic membranes away from the cranial region, the embryonic eye is accessible to stages as late as E7. As we have previously reported, the removal and displacement of the amniochorionic and chorioallantoic membranes, respectively, do not affect embryonic development11. Exposed embryos are viable and are readily amenable to physical manipulation. At this stage, the cornea has three distinct layers (epithelium, stroma, and endothelium), making it suitable for wound healing studies following linear incision into the anterior stroma. It is to be noted that, due to increasing allantois growth over time, access to the eye eventually is occluded at E8. To circumvent this problem, if access to later-stage eyes for wounding is desirable, we have found that access to the eye can be maintained through E9 by carrying out daily manipulation of the extraembryonic membranes (e.g., at E7, E8, etc.), wherein the allantois is carefully repositioned away from the cranial region to ensure that its growth occurs directionally away from the embryo. This enables corneas to be wounded at these later stages (e.g., following innervation).
Overall, embryo viability and survivability in this technique rely on several factors, such as ensuring that a sterile and hydrated egg environment is maintained while further taking caution to not inflict damage on embryonic blood vessels or the allantois. The entire egg surface should be sterilized with ethanol prior to windowing to maintain sterility. This is because small eggshell fragments, which are often laden with microbes, will typically fall into the egg during the windowing procedure. Similarly, all tools involved in windowing, membrane dissections, and making the corneal incision must be thoroughly rinsed in ethanol and dried or flame-sterilized prior to their usage. Moreover, antibiotics should be added to the egg anytime the embryo is exposed to the outside environment. It is further critical that the embryo retains proper hydration post-windowing. Great care must be taken to ensure the window is fully sealed with tape and that no air gaps remain. Given the importance of the tape remaining fully adhered to the eggshell surface and completely sealing the hole, the eggshell surface surrounding the hole needs to be cleaned and dried before applying the tape. Finally, it is imperative that no blood vessels are inadvertently cut and that the allantois, which stores liquid waste from the embryo, is not damaged during the dissection of the extraembryonic membranes as either is lethal to the embryo. It is to be noted that the embryo viability is higher when smaller tears to the chorion and amnion are made, though the tear must be sufficiently large to position the extraembryonic membranes away from the cranial region. If these careful steps are taken during the dissection and removal of membranes from high-quality eggs, one can expect nearly all of the embryos to survive the windowing procedure (~99%), while ~40% of the exposed embryos survive to E9 and ~30% to E1211. In our experience, wounding the corneas of E7-E9 membrane dissected embryos has little impact on embryo viability, and ample embryos remain viable through E18, at which point the cornea wound is fully healed.
Achieving wounds that traverse the corneal epithelium and penetrate the anterior stroma is essential to produce reliable and reproducible results. A high-quality micro-dissecting knife is necessary so that very little pressure needs to be applied. Using a pair of curved iris forceps can be helpful to gently cradle the head as the laceration is made with the other hand. In this manner, the curved iris forceps serve as a backstop so that the cornea remains stationary during the wounding. The penetration of the wound into the stroma is variable, especially when learning, but becomes more reproducible as the researcher learns the feel and look of breaking the corneal epithelium. As one is learning, it may be helpful to view wounded corneas in cross-section so that the depth of wound penetration into the corneal stroma may be assessed (see Figure 3B for an example of a cross-sectioned cornea displaying ideal wounding depth immediately following corneal laceration).
When combined with classical developmental biology techniques, such as tissue grafting and bead implantation, or modern approaches for gene manipulation, such as DNA electroporation and retroviral infection, this animal model of corneal regeneration promises to reveal the molecular factors and cellular mechanisms necessary to achieve complete recovery of the corneal tissue following damage. Further, this animal model could be used to test the potential usefulness of exogenous therapeutic compounds to augment corneal regeneration.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by an Artistic and Scholarly Development grant through Illinois Wesleyan University to TS and funded in part by NIH-R01EY022158 (PL).
Materials
| 18 G hypodermic needle | Fisher Scientific | 14-826-5D | |
| 30 degree angled microdissecting knife | Fine Science Tools | 10056-12 | |
| 4′,6-diamidino-2-phenylindole (DAPI) | Molecular Probes | D1306 | |
| 5 mL syringe | Fisher Scientific | 14-829-45 | |
| Alexa Fluor labelled secondary antibodies | Molecular Probes | ||
| Calcium chloride dihydrate (CaCl2-H20) | Sigma | C8106 | |
| Chicken egg trays | GQF | O246 | |
| Dissecting Forceps, Fine Tip, Serrated | VWR | 82027-408 | |
| Dissecting scissors, sharp tip | VWR | 82027-578 | |
| Iris 1 x 2 Teeth Tissue Forceps, Full Curved | VWR | 100494-908 | |
| Kimwipes | Sigma | Z188956 | |
| Microdissecting Scissors | VWR | 470315-228 | |
| Mouse anti-fibronectin (IgG1) | Developmental Studies Hybridoma Bank | B3/D6 | |
| Mouse anti-laminin (IgG1) | Developmental Studies Hybridoma Bank | 3H11 | |
| Mouse antineuron-specific β-tubulin (Tuj1, IgG2a) | Biolegend | 801213 | |
| Mouse anti-tenascin (IgG1) | Developmental Studies Hybridoma Bank | M1-B4 | |
| Paraformaldehyde | Sigma | 158127 | |
| Penicillin/Streptomycin | Sigma | P4333 | |
| Potassium chloride (KCl) | Sigma | P5405 | |
| Sodium chloride (NaCl) | Fisher Scientific | BP358 | |
| Sportsman 1502 egg incubator | GQF | 1502 | |
| Tear by hand packaging (1.88 inch width) | Scotch | n/a |
Referenzen
- Wilson, S. E. Corneal wound healing. Experimental Eye Research. 197, 108089 (2020).
- Ljubimov, A. V., Saghizadeh, M. Progress in corneal wound healing. Progress in Retinal and Eye Research. 49, 17-45 (2015).
- Whitcher, J. P., Srinivasan, M., Upadhyay, M. P. Corneal blindness: a global perspective. Bulletin of the World Health Organization. 79 (3), 214-221 (2001).
- Ritchey, E. R., Code, K., Zelinka, C. P., Scott, M. A., Fischer, A. J. The chicken cornea as a model of wound healing and neuronal re-innervation. Molecular Vision. 17, 2440-2454 (2001).
- Berdahl, J. P., Johnson, C. S., Proia, A. D., Grinstaff, M. W., Kim, T. Comparison of sutures and dendritic polymer adhesives for corneal laceration repair in an in vivo chicken model. Archives of Ophthalmology. 127 (4), 442-447 (2009).
- Fowler, W. C., Chang, D. H., Roberts, B. C., Zarovnaya, E. L., Proia, A. D. A new paradigm for corneal wound healing research: the white leghorn chicken (Gallus gallus domesticus). Current Eye Research. 28 (4), 241-250 (2004).
- Huh, M. I., Kim, Y. E., Park, J. H. The distribution of TGF-β isoforms and signaling intermediates in corneal fibrotic wound repair. Journal of Cellular Biochemistry. 108 (2), 476-488 (2009).
- Spurlin, J. W., Lwigale, P. Y. Wounded embryonic corneas exhibit nonfibrotic regeneration and complete innervation. Investigative Ophthalmology & Visual Science. 54 (9), 6334-6344 (2013).
- Koudouna, E., Spurlin, J., Babushkina, A., Quantock, A. J., Jester, J. V., Lwigale, P. Y. Recapitulation of normal collagen architecture in embryonic wounded corneas. Scientific Reports. 10 (1), 13815 (2020).
- Luo, J., Redies, C. Ex ovo electroporation for gene transfer into older chicken embryos. Developmental Dynamics. 233 (4), 1470-1477 (2005).
- Spurlin, J., Lwigale, P. Y. A technique to increase accessibility to late-stage chick embryos for in ovo manipulations. Developmental Dynamics. 242 (2), 148-154 (2013).
- Hamburger, V., Hamilton, H. L. A series of normal stages in the development of the chick embryo. Journal of Morphology. 88 (1), 49-92 (1951).
- Neath, P., Roche, S. M., Bee, J. A. Intraocular pressure dependent and independent growth phases of the embryonic chick eye and cornea. Investigative Ophthalmology & Visual Science. 32 (9), 2483-2491 (1991).
- Matsuda, A., Yoshiki, A., Tagawa, Y., Matsuda, H., Kusakabe, M. Corneal wound healing in tenascin knockout mouse. Investigative Ophthalmology & Visual Science. 40 (6), 1071-1080 (1990).
- Nishida, T., Nakagawa, S., Nishibayashi, C., Tanaka, H., Manabe, R. Fibronectin enhancement of corneal epithelial wound healing of rabbits in vivo. Archives of Ophthalmology. 102 (3), 455-456 (1984).
- Sumioka, T., et al. Impaired cornea wound healing in a tenascin C-deficient mouse model. Lab Investigation. 93 (2), 207-217 (2013).
- Tervo, K., van Setten, G. B., Beuerman, R. W., Virtanen, I., Tarkkanen, A., Tervo, T. Expression of tenascin and cellular fibronectin in the rabbit cornea after anterior keratectomy. Immunohistochemical study of wound healing dynamics. Investigative Ophthalmology & Visual Science. 32 (11), 2912-2918 (1991).
- Lwigale, P. Y., Bronner-Fraser, M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Entwicklungsbiologie. 306 (2), 750-759 (2007).
- Kubilus, J. K., Linsenmayer, T. F. Developmental corneal innervation: interactions between nerves and specialized apical corneal epithelial cells. Investigative Ophthalmology & Visual Science. 51 (2), 782-789 (2010).
- Schwend, T., Deaton, R. J., Zhang, Y., Caterson, B., Conrad, G. W. Corneal sulfated glycosaminoglycans and their effects on trigeminal nerve growth cone behavior in vitro: roles for ECM in cornea innervation. Investigative Ophthalmology & Visual Science. 53 (13), 8118-8137 (2012).
- Lee, M. K., Tuttle, J. B., Rebhun, L. I., Cleveland, D. W., Frankfurter, A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motility and the Cytoskeleton. 17 (2), 118-132 (1990).
- Chen, X., Nadiarynkh, O., Plotnikov, S., Campagnola, P. J. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nature Protocols. 7, 654-669 (2012).
- Campagnola, P. J., Millard, A. C., Terasaki, M., Hoppe, P. E., Malone, C. J., Mohler, W. A. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophysical Journal. 82 (1), 493-508 (2002).
- Cloney, K., Franz-Odendaal, T. A. Optimized ex-ovo culturing of chick embryos to advanced stages of development. Journal of Visualized Experiments. (95), e52129 (2015).
- Waldvogel, J. A. The bird’s eye view. American Scientist. 78, 342-353 (1990).
- Martin, P., Parkhurst, S. M. Parallels between tissue repair and embryo morphogenesis. Development. 131 (13), 3021-3034 (2004).
- Wilson, S. E., Mohan, R. R., Mohan, R. R., Ambrosio, R., Hong, J., Lee, J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Progress in Retinal and Eye Research. 20 (5), 625-637 (2001).

