Differentiation of Monocytes into Phenotypically Distinct Macrophages After Treatment with Human Cord Blood Stem Cell (CB-SC)-Derived Exosomes
Summary
Exosome application is an emerging tool for drug development and regenerative medicine. We establish an exosome isolation protocol with high purity to isolate exosomes from novel identified stem cells called CB-SC for mechanistic studies. We also coculture CB-SC-derived exosomes with human monocytes, leading to their differentiation into phenotypically distinct macrophages.
Abstract
Stem Cell Educator (SCE) therapy is a novel clinical approach for the treatment of type 1 diabetes and other autoimmune diseases. SCE therapy circulates the isolated patient’s blood mononuclear cells (e.g., lymphocytes and monocytes) through an apheresis machine, co-cultures the patient’s blood mononuclear cells with adherent cord blood-derived stem cells (CB-SC) in the SCE device, and then returns these “educated” immune cells to the patient’s blood. Exosomes are nano-sized extracellular vesicles between 30‒150 nm existing in all biofluid and cell culture media. To further explore molecular mechanisms underlying SCE therapy and determine the actions of exosomes released from CB-SC, we investigate which cells phagocytize these exosomes during the treatment with CB-SC. By co-culturing Dio-labeled CB-SC-derived exosomes with human peripheral blood mononuclear cells (PBMC), we found that CB-SC-derived exosomes were predominantly taken up by human CD14-positive monocytes, leading to the differentiation of monocytes into type 2 macrophages (M2), with spindle-like morphology and expression of M2-associated surface molecular markers. Here, we present a protocol for the isolation and characterization of CB-SC-derived exosomes and the protocol for the co-culture of CB-SC-derived exosomes with human monocytes and the monitoring of M2 differentiation.
Introduction
Cord blood stem cells (CB-SC) are unique type of stem cells identified from human cord blood and are distinguished from other known types of stem cells such as mesenchymal stem cells (MSC) and hematopoietic stem cells (HSC)1. Based on their unique properties of immune modulation and their ability to tightly adhere to the surface of Petri dishes, we developed a new technology designated as Stem Cell Educator (SCE) therapy in clinical trials2,3. During SCE therapy, a patient’s peripheral blood mononuclear cells (PBMC) are collected and circulated through a cell separator and co-cultured with adherent CB-SC in vitro. These “educated” cells (CB-SC-treated PBMC) are then returned to the patient’s circulation in a closed-loop system. Clinical trials have already demonstrated the clinical safety and efficacy of SCE therapy for the treatment of autoimmune diseases including type 1 diabetes (T1D)2,4 and alopecia areata (AA)5.
Exosomes are a family of nanoparticles with diameters ranging 30‒150 nm and exist in all biofluid and cell culture media6. Exosomes are enriched with many bioactive molecules including lipids, mRNAs, proteins, and microRNAs (miRNA), and play an important role in cell-to-cell communications. Of late, exosomes have become more attractive for researchers and pharmaceutical companies due to their therapeutic potentials in clinics7,8,9. Recently, our mechanistic studies demonstrated that CB-SC-released exosomes contribute to the immune modulation of SCE therapy10.
Here, we describe the protocol to explore the mechanism of SCE therapy targeting monocytes by CB-SC-released exosomes. First, CB-SC-released exosomes were isolated from CB-SC-derived conditioned media using ultracentrifugation methods and validated by flow cytometry, western blot (WB) and dynamic light scattering (DLS). Second, CB-SC-derived exosomes were labeled with a green fluorescent lipophilic dye: Dio. Third, they were co-cultured with PBMC to examine the positive percentages of Dio-labeled CB-SC-derived exosomes at the different subpopulations of PBMC by flow cytometry. This protocol provides guidance to study the action of exosomes underlying the immune modulation of stem cells.
Protocol
The protocol follows the guidelines of institutional human research ethics committee at Center for Discovery and Innovation, Hackensack Meridian Health. Human buffy coat blood units were purchased from the New York Blood Center (New York, NY). Human umbilical cord blood units were collected from healthy donors and purchased from Cryo-Cell International blood bank (Oldsmar, FL). Both New York Blood Center and Cryo-Cell have received all accreditations for blood collections and distributions, with IRB approval and signed Consent Forms from donors.
1. Cell culture and preparation of CB-SC-derived conditioned medium
- Transfer 25 mL of cord blood (Table of Materials) over 20 mL of density gradient medium (γ = 1.077) into a 50 mL conical tube.
- Centrifuge at 1,690 x g for 20 min at 20 °C in a swinging-bucket rotor without brake.
- Carefully transfer the mononuclear cell layer (buffy coat) to a new 50 mL conical tube. Fill the conical tube with phosphate buffered saline (PBS) to 40 mL. Mix and centrifuge to pellet cells at 751 x g for 10 min at 20 °C.
- Discard the supernatant and add 15 mL of ACK lysis buffer (Table of Materials) to the cell pellet. Re-suspend cells through pipetting. Then incubate for 10 min at room temperature.
NOTE: This step removes the red blood cells. - Fill the conical tube with 25 mL of PBS. Centrifuge at 751 x g for 5 min and discard supernatant to obtain pelleted mononuclear cells.
- Wash 2x with 40 mL of PBS to remove the remaining lysis buffer.
- Centrifuge at 751 x g for 5 min to pellet the cells.
- Discard the supernatant and re-suspend cord blood mononuclear cells with 10 mL of chemical-defined serum-free medium (Table of Materials) per tube.
- Combine cord blood mononuclear cells to one tube.
- Take 20 µL cell suspension and mix with 20 µL of 0.4% trypan blue solution (Table of Materials) in a 1.5 mL tube.
- Load into the chamber slide and quantify the cell number and cell viability with an automated cell counter.
NOTE: Cell suspension is diluted at 1:10 if cell concentration is above 1 x 107 cells/mL. - Seed mononuclear cells in 150 mm x 15 mm Petri dishes at 1 x 106 cells/mL, 25 mL/dish in chemical-defined serum-free cell culture medium.
- Incubate at 37 °C under 8% CO2 conditions for 10‒14 days until CB-SC reach more than 80% confluence.
- Discard the supernatant and wash with 15 mL of PBS per Petri dish; then, remove the PBS.
NOTE: CB-SC are attached to Petri dishes tightly. - Repeat step 1.14 two times.
- Add 25 mL of chemical-defined serum free medium per Petri dish.
- Incubate at 37 °C under 8% CO2 conditions for 3‒4 days.
- Collect the CB-SC-derived conditioned medium into 50 mL conical tubes.
2. Characterization of CB-SC
- Detach CB-SC by pipetting 10 mL of PBS-based cell dissociation buffer up and down with a 5 mL pipette tip (Table of Materials).
- Centrifuge at 1,690 x g for 5 min to pellet cells and re-suspend in 200 µL of PBS.
- Fix and permeabilize cells for intracellular staining via staining preparation kit (Table of Materials).
- Add 5 µL of Fc blocker (Table of Materials) per sample and incubate for 15 min at room temperature.
NOTE: Fc blocker inhibits non-specific binding when staining with antibodies. - Add fluorescence-conjugated mouse anti-human monoclonal antibodies including CD34, CD45, SOX2, OCT3/4, CD270, and Galectin 9 at 25 µg/mL (Table of Materials) to 100 μL volume of cells. Incubate for 30 min at room temperature with light protection.
- After staining, wash cells with 1 mL of PBS and centrifuge at 751 x g for 10 min to pellet cells.
- Re-suspend cells with 200 μL of PBS and transfer into a 5 mL tube.
- Perform flow cytometry to validate the expression of CB-SC-associated above specific markers.
3. Isolation of CB-SC-derived exosomes
- Centrifuge the conditioned medium collected from step 1.18 at 300 x g for 10 min at 4 °C. Transfer the supernatant to a new 50 mL conical tube.
- Centrifuge the supernatant collected from step 3.1 at 2,000 x g for 20 min at 4 °C. Transfer the supernatant to a new 50 mL conical tube.
- Centrifuge the supernatant collected from step 3.2 at 10,000 x g for 30 min at 4 °C. Transfer the supernatant to a new 50 mL conical tube.
NOTE: The fixed angle rotor is used so that the cell pellets are precipitated to the side of the tube. Mark the side of the cap and draw a circle on the side of the tube where the pellet is expected. - Filter supernatants collected from step 3.3 with a 0.22 µm filter (Table of Materials).
- Transfer 15 mL of media to each 10 kDa centrifugal filter unit (Table of Materials).
- Centrifuge at 4,000 x g for 30 min to isolate the concentrated exosome media.
- Transfer the concentrated exosomes to an ultracentrifuge tube. Then, pellet exosomes at 100,000 x g for 80 min at 4 °C.
- Discard the supernatant and re-suspend the pellet exosomes in 10 mL of PBS.
- Centrifuge at 100,000 x g for 80 min at 4 °C to collect the exosomes pellet.
- Re-suspend the exosomes pellet in 200 µL of PBS by pipetting up and down.
4. Characterization of CB-SC-derived exosomes
- Quantifying total protein concentration of exosome preparation by bicinchoninic acid assay (BCA) kit
- Pipette 10 µL of each albumin standard and isolated exosome sample prepared in step 3.10 into a 96-well plate in duplicate.
- Add 200 µL of the working reagent from the BCA kit to each well. Mix contents of the plate thoroughly on the plate shaker for 10 s.
NOTE: Working reagent (WR): 50-part reagent A with 1-part reagent B. - Cover the plates with foil and incubate them at 37 °C for 30 min.
- Cool the plates to room temperature (RT).
- Measure sample absorbance at 562 nm via a plate reader.
- Preparation and staining of exosomes for flow cytometry
- Capture exosomes by adding 20 μL of anti-human CD63 magnetic beads (4.5 μm size) (Table of Materials) into 25 μg of CB-SC-derived exosomes prepared in step 3.10 in total 100 μL volume of PBS.
- Incubate the tube overnight (18‒22 h) at 4 °C on the shaker at 800 rpm.
- Centrifuge the tube at 300 x g for 30 s to collect the sample at the bottom of the tube.
- Add 300 µL of isolation buffer (0.1% bovine serum albumin (BSA) in PBS) and mix gently by pipetting.
NOTE: This step washes the bead-bound exosomes. - Place the tube on a magnet stand for 1 min (Table of Materials) and discard the supernatant.
- Repeat steps 4.2.4‒4.2.5.
- Re-suspend the bead-bound exosomes with 400 µL of isolation buffer.
- Aliquot 100 µL of bead-bound exosomes to each tube.
- Add fluorescence-conjugated antibodies (CD9-FITC, CD81-PE, and CD63-FITC at 25 µg/mL, respectively) to each flow tube with CD63 bead-captured exosomes.
NOTE: Isotype-matched IgGs serve as negative controls. - Incubate for 45 min at room temperature with light protection on the shaker at 800 rpm.
- Repeat steps 4.2.4‒4.2.5.
- Re-suspend the bead-bound exosomes in 200 µL of isolation buffer and transfer to 5 mL flow tubes.
- Place the tubes in the sample carousel of the flow cytometer.
- Open the protocol for exosome testing.
- Run the sample automatically by flow cytometer.
- Exosome detection by western blot
NOTE: Western blot is a well-established method and we will not go into details of the method itself.- Lyse the pellets of CB-SC-derived exosomes from step 3.10 with 100 µL of RIPA buffer, pipette 20x, then place on ice for 5 min.
- Quantify the protein concentration of exosome lysate by BCA kit and load 25 µg of protein per well.
- Separate the proteins by gel electrophoresis for 40 min at 150 V.
- Transfer the protein to polyvinylidene fluoride (PVDF) membrane using semi-dry transferring method11.
- Block the membrane with 5% non-fat milk for 30 min.
- Incubate with 2 µg/mL anti-human Alix (Table of Materials) and 1 µg/mL anti-human Calnexin antibodies (Table of Materials).
- Detect the protein by chemiluminescence with a digital imaging system.
- Exosome validation by dynamic light scattering (DLS)
- Dilute 10 µg of CB-SC-derived exosome samples in 1 mL of PBS.
- Transfer the 1 mL of diluted sample into disposable semi-micro cuvette (Table of Materials).
- Place the cuvette in the DLS instrument. Set the refractive index (RI) as 1.39 for all the sample monitor.
- Run samples at 25 °C and acquire three measurements per fraction to get an average size distribution.
- Exosome validation by transmission electron microscopy (TEM)
- Coat formvar on 300 mesh copper grids12 (Table of Materials).
- Strengthen the formvar with the additional layer of evaporated carbon on copper grids12.
NOTE: Such a coating approach is excellent for specimen support. - Load 10 µL of exosome samples onto grids and leave to air-dry.
- Negatively stain samples with uranyl acetate for 5 min.
- Wash three times with DI water and leave to air-dry.
- Observe and photograph the samples under TEM. Set the accelerating voltage at 200 kV and spot size at 2.
5. Measure Dio-labeled CB-SC-derived exosomes up taken by different subpopulations of PBMC
- Label CB-SC-derived exosomes with green fluorescent lipophilic dye Dio
- Transfer 100 µg of CB-SC-derived exosomes (prepared in step 3.10) into a 15 mL centrifuge tube.
- Dilute sample with PBS to 5 mL.
- Add green fluorescent lipophilic dye Dio (Table of Materials) until working concentration reaches 5 µM.
- Incubate for 15 min at room temperature protected from light.
- Transfer the sample into an ultracentrifuge tube.
- Centrifuge at 100,000 x g for 80 min to pellet Dio-labeled CB-SC-derived exosomes.
- Re-suspend the labeled exosomes in 200 µL of PBS.
- Preparation of human PBMC
- Transfer 25 mL of human buffy coat (Table of Materials) over 20 mL of density gradient medium (γ = 1.077) into a 50 mL conical tube.
- Repeat steps 1.2 to 1.11.
- Transfer 1 x 106 PBMC into a non-tissue-treated hydrophobic 24-well plate (1 mL/well).
NOTE: A non-tissue-treated plate was used to avoid adhering of monocytes.
- Co-culture Dio-labeled exosomes with PBMC
- Transfer 40 µL Dio-labeled CB-SC-derived exosomes prepared in step 5.1.7 to each PBMC-containing well in a 24-well plate using a 200 µL pipette. Add the same volume of PBS to control wells.
- Mix by pipetting 10x. Incubate for 4 h.
- Collect 200 μL exosome-treated PBMC and label with Hoechst 33342 for 10 min at room temperature.
- Centrifuge at 300 x g for 10 min at room temperature. Discard the supernatant and re-suspend the cell pellet in 100 µL of PBS.
- Mount cells onto microscope slides.
- Observe and photograph the interaction of Dio-labeled CB-SC-derived exosomes with Hoechst 33342-labeled PBMC using a microscope.
- Transfer the remaining cells from step 5.3.3 into a 1.5 mL tube.
- Centrifuge at 300 x g for 10 min at 4 °C. Discard the supernatant and re-suspend the cell pellet in 200 µL of PBS.
- Add 5 µL of Fc blocker per sample. Incubate for 15 min at room temperature.
- Add antibodies (CD3, CD4, CD8, CD11c, CD14, CD19, and CD56 at 25 µg/mL) (Table of Materials) to stain PBMC.
NOTE: Isotype-matched IgGs serve as negative controls. - Incubate for 30 min at room temperature with light protection.
- Add 1 mL of PBS and centrifuge at 300 x g for 10 min at 4 °C to pellet the cells.
- Re-suspend the cells with 200 µL PBS. Add 5 µL of propidium iodide.
- Use flow cytometry to evaluate the level of Dio-labeled exosome uptake in different subpopulation of PBMC.
6. Examine the action of CB-SC-derived exosomes on monocytes
- Isolation human CD14-positive monocytes
- Transfer 3 x 107 human PBMC into a 15 mL tube.
- Centrifuge at 300 x g for 10 min at 4 °C.
- Place the separation column (Table of Materials) in the magnet separator (Table of Materials).
- Wash separation columns three times with 2 mL of cold running buffer (Table of Materials).
- Re-suspend the cells in 300 µL of cold PBS. Add 60 µL of CD14 microbeads. Mix well and incubate on ice for 15 min.
- Add 6 mL of cold PBS. Centrifuge at 300 x g for 10 min at 4 °C.
- Re-suspend the pelleted cells in 500 µL of cold running buffer.
- Transfer cells into the separation column (prepared in step 6.14) and let them pass through.
- Wash the separation column three times with 2 mL of running buffer per wash. Lift the column from the magnet separator and place it in a 15 mL centrifuge tube.
NOTE: The 15 mL tube should be placed on ice due to the adherence of CD14-positive monocytes to the tube at room temperature. - Transfer 2 mL of cold running buffer to the top of the column and isolate the CD14-positive cells into the 15 mL tube.
- Centrifuge at 300 x g for 10 min at 4 °C to pellet the CD14-positive cells.
- Re-suspend the cells with 2 mL of cold chemical-defined serum free medium (Table of Materials).
- Transfer 50 µL of cells into a 1.5 mL tube.
- Stain with 10 µL of Krome Orange-conjugated anti-human CD14 mAb (Table of Materials) for 20 min.
NOTE: Isotype-matched IgGs serve as negative controls. - Add 1 mL PBS to the cells. Centrifuge at 300 x g for 10 min to pellet cells.
- Re-suspend cells in 200 µL of PBS and transfer it to a 5 mL tube. Determine the purity of CD14-positive monocytes by flow cytometry.
- Treatment of monocytes with CB-SC-derived exosomes
- Seed 1 x 106 purified monocytes with chemical-defined serum-free culture medium (Table of Materials) in tissue culture-treated 6-well plate (2 mL/well).
- Incubate for 2 h at 37 °C under 5% CO2.
- Discard the supernatant with 1 mL pipette. Add 2 mL of 37 °C pre-warmed chemical-defined serum-free culture medium (Table of Materials) gently.
NOTE: Monocytes were adhered to the plate within 2 h. Floating cells were identified as dead or other cell contaminations. - Add 80 µg CB-SC-derived exosomes isolated from step 3.10 to monocyte cultures in a 6-well plate with total volume of 2 mL.
NOTE: The same volume of PBS was added to control wells. - Incubate at 37 °C under 5% CO2 for 3‒4 days.
- Photograph the cell morphology using an inverted microscope at 200× magnification (Table of Materials).
- Detach cells by pipetting up and down in 1 mL of a PBS-based cell dissociation buffer with 1 mL pipette tip.
- Harvest the remaining attached cells via a cell scraper.
NOTE: Since primary monocytes or differentiated macrophages attach tightly, some cells remain adhered to the bottom after the treatment with dissociation buffer. Therefore, these cells are harvested with a cell scraper. - Collect cells at 1,690 x g for 5 min. Re-suspend cells in 200 µL of PBS.
- Add 5 µL of Fc blocker (25 µg/mL) to block non-specific binding.
- Add antibodies (CD14, CD80, CD86, CD163, CD206, and CD209 at 25 µg/mL, Table of Materials) to cells. Incubate for 30 min at room temperature.
NOTE: Isotype-matched IgGs serve as negative control - Add 1 mL of PBS to cells and centrifuge at 300 x g for 10 min. Discard the supernatant and re-suspend with 200 µL of PBS.
- Add 5 µL of propidium iodide per sample (200 µL) and transfer cells to a new 5 mL flow tube.
- Perform the flow cytometry and evaluate the levels of CD14, CD80, CD86, CD163, CD206, and CD209 expressions.
Representative Results
Initially, the phenotype and purity of CB-SC were examined by flow cytometry with CB-SC-associated markers such as leukocyte common antigen CD45, ES cell-specific transcription factors OCT3/4, and SOX2. CB-SC display high levels of CD45, OCT3/4, SOX2, CD270, and galectin 9 expression, but no expression of CD34 (Figure 1A). Flow cytometry analysis confirmed the expression of exosome-specific markers including CD9, CD81, and CD63 were on CB-SC-derived exosomes (Figure 1B). Morphology and size distribution of exosomes were characterized by TEM and DLS (Figure 1C,D), with the size of 79.38 ± 20.07 nm. Western blot further proved the expression of the exosome-associated marker Alix, without expression of the ER-associated marker Calnexin (Figure 1E).
PBMC were treated with Dio-labeled CB-SC-Exo. The microscopy observation demonstrated the direct interaction of Dio-labeled CB-SC-Exo with PBMC (Figure 2A). To better define which cell population interacted with the Dio-labeled CB-SC-Exo, different cell compartments were gated with cell-specific markers such as CD3 for T cells, CD11c for myeloid dendritic cells (DC), CD14 for monocytes, CD19 for B cells, and CD56 for NK cells (Figure 2B). After an incubation for 4 hr, flow cytometry demonstrated that different blood cell compartments displayed at different median fluorescence intensity (MFI) of Dio-positive exosomes (Figure 2C). Notably, monocytes exhibited higher median fluorescence intensity of Dio-positive CB-SC-Exo than those of other immune cells (Figure 2C), highlighting that monocytes were primarily targeted by the CB-SC-derived exosomes.
To explore the direct effects of CB-SC-derived exosomes on monocytes, the purified CD14+ monocytes were co-cultured with CB-SC-derived exosomes for 3 days. The exosome-treated monocyte successfully differentiated into spindle-like morphologies (Figure 3A). Next, phenotypes of the CB-SC-Exo treated or untreated monocytes were tested, revealing the expressions of M2-associated markers including CD163, CD206, CD209 were markedly increased among the exosome-treated group (Figure 3B, red histogram). Comparing with the conventional M2 macrophages generated by M-CSF + IL-4, CB-SC-Exo-treated monocytes expressed similar levels of M2-associated markers such as CD163, CD206, CD209, with no significant differences (Figure 3C). Therefore, the data indicates that monocytes differentiate into macrophages with M2 phenotype after the treatment with CB-SC-derived exosomes.
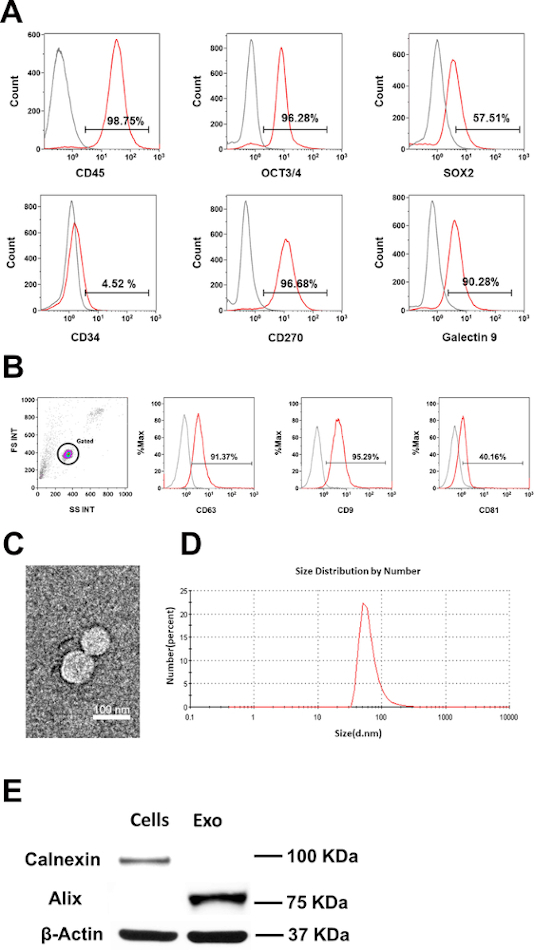
Figure 1: Characterization of CB-SC-derived exosomes. (A) Phenotypic characterization of CB-SC, high expression of CD45, OCT3/4, SOX2, CD270 and Galectin-9, no expression of CD34. (B) Expressions of exosome-associated markers (CD63, CD9, CD81) on CB-SC-derived exosomes. Isotype-matched IgGs served as controls for flow cytometry (gray histogram). (C) Transmission Electron Microscopy (TEM) image of the CB-SC-derived exosomes. (D) Size distribution of CB-SC-derived exosomes using Dynamitic Light Scattering (DLS). (E) Western blots show that CB-SC-derived exosomes display the exosome-specific marker Alix, but negative for endoplasmic reticulum (ER)-associated marker Calnexin. Please click here to view a larger version of this figure.
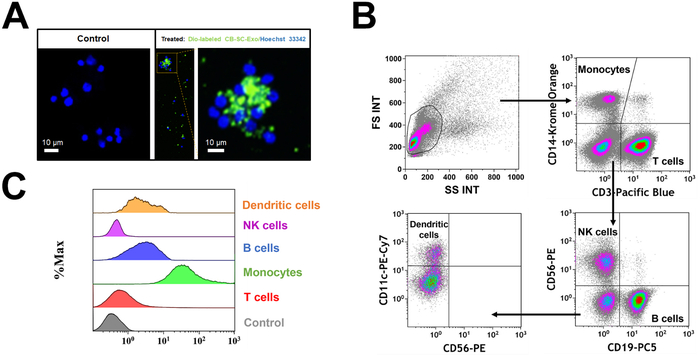
Figure 2: Interaction of CB-SC-derived exosomes with different populations of PBMC. (A) The interaction of Dio-labeled CB-SC-Exo (green) with PBMC (blue, nuclear staining with Hoechst 33342) was photographed with Nikon Eclipse Ti2 microscope with NIS-Elements software version 5.11.02, with a high magnification showing the distribution of Dio-labeled exosomes (green) in the PBMC cells after the co-incubation for 4 h 5% CO2 in the non-tissue culture-treated 24-well plate. n = 2. (B) Gating strategy for flow cytometry analysis with cell-specific surface markers for different subpopulations in PBMC, including CD3 for T cells, CD14 for monocytes, CD19 for B cells, CD56 for NK cells, and CD11c for DCs. (C) Display different median fluorescence intensity (MFI) of Dio-labeled exosome among different PBMC subpopulations (e.g., T cells, Monocytes, B cells, NK cells, DCs). Please click here to view a larger version of this figure.
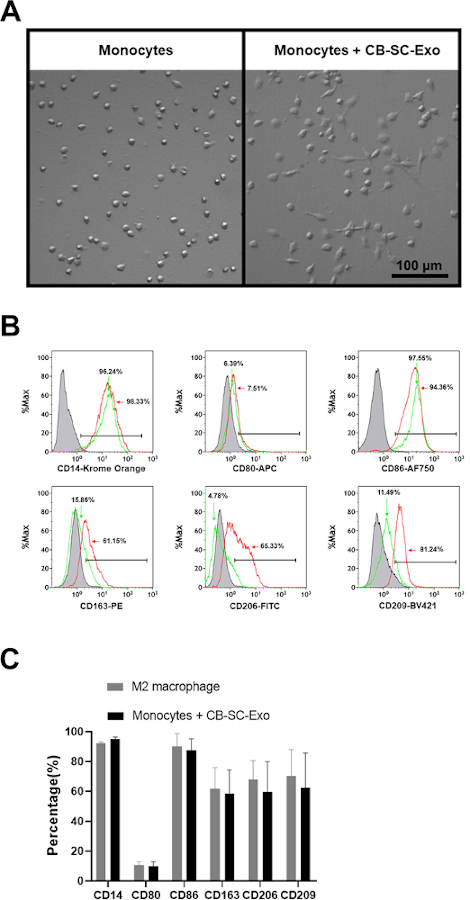
Figure 3: Effects of CB-SC-derived exosomes on monocytes. (A) Morphological change of monocytes into the spindle-like cells after treatment with CB-SC-derived exosomes. (B) Up-regulated the level of M2-associate markers’ expression after the treatment with CB-SC-derived exosomes, such as CD163, CD206, and CD209 (red line). Untreated monocytes (green line) served as control. Isotype-matched IgGs served as negative controls (gray line). (C) Phenotypic comparison between conventional M2 macrophages and the CB-SC-Exo-induced M2 macrophages. To generate the conventional M2 macrophages, the purified CD14+ monocytes were treated with 50 ng/mL macrophage colony-stimulating factor (M-CSF) at 37 °C, 5% CO2 conditions for 7 days, and followed by the overnight treatment with 10 ng/mL IL-4. M2-associated markers including CD14. CD80, CD86, CD163, CD206, and CD209 were evaluated by flow cytometry. Isotype-matched immunoglobulin G (IgG) serve as control. The data is presented as mean ± SD; N = 3. Please click here to view a larger version of this figure.
Discussion
Application of exosomes is an emerging field for clinical diagnosis, drug developments and regenerative medicine. Here, we present a detailed protocol regarding the preparation of CB-SC-derived exosomes and the functional study of exosomes on the differentiation of human monocytes. The current protocol demonstrated that functional CB-SC-derived exosomes are isolated by sequential centrifugation and ultracentrifugation with high purity and exhibiting the immune modulation on monocytes.
As compared with other conventional protocols, ultrafiltration is an established approach for the isolation and purification of exosomes from different cells or media, based upon the molecular weight and exclusion sizes that are different from other extracellular vesicles (EVs). While ultrafiltration isolation is more time-saving than the ultracentrifugation-based separation, it may cause structural damage to vesicles at large sizes. Exosomes can also be collected by polyethylene glycol (PEG)-mediated precipitation at low cost, though this method risks the exosome purity due to the protein contaminations13,14. Therefore, the current protocol was cost-effective to produce exosomes at high purity. Based on the immune modulations of CB-SC-derived exosomes10, characterization of CB-SC-derived exosomes may offer a valuable biomarker to evaluate the potency of Stem Cell Educator with CB-SC before clinical applications.
Macrophages are professional antigen-presenting cells against viral and bacterial infections, with varied biological functions and heterogeneities. Based on their differences in surface markers and immune function, macrophages are categorized with two sub-populations: type 1 macrophages (M1, conventional macrophages causing inflammation) and type 2 macrophages (M2, displaying anti-inflammation)15. This study established that purified human monocytes were differentiated into type 2 macrophages after the treatment with CB-SC-derived exosomes, displaying an anti-inflammation phenotype10. CB-SC-derived exosome-treated monocytes exhibited the elongated morphology and expressed the M2-associated surface markers (e.g., CD163, CD206, and CD209), with the similar phenotype as the conventional M2 macrophages generated using cytokines M-CSF + IL-4. Such phenotypic changes of monocytes highlight the new mechanism underlying the immune modulation of CB-SC for the treatment of type 1 diabetes and other autoimmune diseases. During the SCE therapy, patients’ immune cells were co-cultured with CB-SC for around 8‒9 h. The SCE-treated monocytes carried the CB-SC-derived exosomes back into the body, which contributed to the M2 differentiation and the expansion of the induction of immune tolerance, leading to the improvement of clinical outcomes after the treatment with SCE therapy.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We are grateful to Mr. Poddar and Mr. Ludwig for generous funding support via Hackensack UMC Foundation. We appreciate Laura Zhao for English editing.
Materials
| 1.5 ml Microcentrifuge tube | Fisher Scientific | 05-408-129 | |
| 15 ml conical tube | Falcon | 352196 | |
| 24-well plate | Falcon | 351147 | Non-tissue culture plate |
| 3,3'-Diostadecyloxacarbocyanine perchlorate(Dio) | Millipore sigma | D4292-20MG | Store at 4 °C |
| 300 Mesh Grids | Ted Pella | 1GC300 | |
| 50 mL conical tube | Falcon | 352070 | |
| 6-well plate | Falcon | 353046 | Tissue culture plate |
| 96-well plate | Falcon | 353072 | Tissue culture plate |
| ACK Lysis Buffer | Lonza | 10-548E | 100 ml |
| Amicon-15 10kDa Centrifuge Fliter Unit | Millipore sigma | UFC901024 | |
| Anti-Human Alix | Biolegend | 634501 | store at 4 °C, RRID: AB_2268110 |
| Anti-Human Calnexin | Biolegend | 699401 | store at 4 °C, RRID: AB_2728519 |
| Anti-Human CD11c antibody, Pe-Cy7 | BD Bioscience | 561356 | store at 4 °C, RRID: AB_10611859 |
| Anti-Human CD14 antibody, Karma Orange | Beckman Coulter | B36294 | store at 4 °C, RRID: AB_2728099 |
| Anti-Human CD163 antibody, PE | BD Bioscience | 556018 | store at 4 °C, RRID: AB_396296 |
| Anti-Human CD19 antibody, PC5 | Beckman Coulter | IM2643U | store at 4 °C, RRID: AB_131160 |
| Anti-Human CD206 antibody, FITC | BD Bioscience | 551135 | store at 4 °C, RRID: AB_394065 |
| Anti-Human CD209 antibody, Brilliant Violet 421 | BD Bioscience | 564127 | store at 4 °C, RRID: AB_2738610 |
| Anti-Human CD270 antibody, PE | Biolegend | 318806 | store at 4 °C, RRID: AB_2203703 |
| Anti-Human CD3 antibody, Pacfic Blue | Biolegend | 300431 | store at 4 °C, RRID: AB_1595437 |
| Anti-Human CD34 antibody, APC | Beckman Coulter | IM2427U | store at 4 °C, RRID: N/A |
| Anti-Human CD4 antibody, APC | BD Bioscience | 555349 | store at 4 °C, RRID: AB_398593 |
| Anti-Human CD45 antibody, Pe-Cy7 | Beckman Coulter | IM3548U | store at 4 °C, RRID: AB_1575969 |
| Anti-Human CD56 antibody, PE | Beckman Coulter | IM2073U | store at 4 °C, RRID: AB_131195 |
| Anti-Human CD63 antibody,PE | BD Bioscience | 561925 | store at 4 °C, RRID: AB_10896821 |
| Anti-Human CD8 antibody, APC-Alexa Fluor 750 | Beckman Coulter | A94686 | store at 4 °C, RRID: N/A |
| Anti-Human CD80 antibody, APC | Beckman Coulter | B30642 | store at 4 °C, RRID: N/A |
| Anti-Human CD81 antibody, FITC | BD Bioscience | 561956 | store at 4 °C, RRID: AB_394049 |
| Anti-Human CD86 antibody, APC-Alexa Fluor 750 | Beckman Coulter | B30646 | store at 4 °C, RRID: N/A |
| Anti-Human CD9 antibody, FITC | ThermoScientic | MA5-16860 | store at 4 °C, RRID: AB_2538339 |
| Anti-Human Galectin 9 antibody, Brilliant Violet 421 | Biolegend | 348919 | store at 4 °C, RRID: AB_2716134 |
| Anti-Human OCT3/4 antibody, eFluor660 | ThermoScientic | 50-5841-82 | store at 4 °C, RRID: AB_11218882 |
| Anti-Human SOX2 antibody, Alexa Fluor 488 | ThermoScientic | 53-9811-82 | store at 4 °C, RRID: AB_2574479 |
| BCA Protein Assay Kit | ThermoFisher Scientific | 23227 | |
| Bovine Serum Albumin | Millipore Sigma | A1933 | |
| Buffy coat | New York Blood Center | 40-60 ml/unit | |
| Cell scraper | Falcon | 353085 | |
| Disposable semi-micro cuvette | VWR | 97000590 | |
| Dissociation buffer | Gibco | 131510014 | 100 ml |
| Dual-Chanber cell counting slides | Bio-Rad | 1450015 | |
| Exosome-Human CD63 Detection reagent | ThermoFisher Scientific | 10606D | store at 4 °C |
| Ficoll-Paque PLUS density grandient media | GE Health | 17-1440-03 | 500 ml |
| Fixed-Angle Rotor(25°) | Thermo Scientifc | 75003698 | Maxium 24,700 x g |
| Gallios flow cytometer | Beckman Coulter | 3 lasers 10 Color Max |
|
| Human cord blood | Cryo-Cell International | 40-100 ml/unit | |
| Human Fc Block | BD Bioscience | 564220 | store at 4 °C |
| Immun-Blot PVDF membrane | Bio-Rad | 1620177 | |
| Millex-GP Syringe Filter Unit, 0.22 µm | Millipore Sigma | SLGP033RS | |
| Optima XE-90 Ultracentriguge | Beckman Coulter | ||
| Orbital Shaker MP4 | BioExpress | S-3500-1 | |
| PBS | ThermoFisher Scientific | 10010049 | 500 ml |
| Propidium Iodide | BD Bioscience | 56-66211E | store at 4 °C |
| Nikon Eclipse Ti2 microscope | Nikon instruments Inc | Eclipse Ti2 | NIS-Elements software version 5.11.02 |
| Hochest 33342 | Thermo Scientifc | 62249 | 5 ml |
| Revert microsopy | Fisher scientific | 12563518 | |
| Rotor 41 Ti | Beckman Coulter | 331362 | Maxium 41,000 rpm |
| Sorvall St 16R Centrifuge | Thermo Scientifc | 75004381 | |
| Swinging Bucket Rotor | Thermo Scientifc | 75003655 | |
| TC-20 cell counter | Bio-Rad | ||
| ThermoScientific Forma 380 Steri Cycle CO2 Incubator | ThermoFisher Scientific | ||
| Transmission electron microscopy | JEOL | JEM-2100 PLUS | |
| Ultracentrifuge tube | Beckman Coulter | 331372 | |
| X'VIVO 15 Serum-free medium | Lonza | BEBP04-744Q | 1000 ml Culture medium, store at 4 °C |
Referenzen
- Zhao, Y. Stem cell educator therapy and induction of immune balance. Current Diabetes Reports. 12 (5), 517-523 (2012).
- Zhao, Y., et al. Reversal of type 1 diabetes via islet beta cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Medicine. 10 (1), 3 (2012).
- Zhao, Y., et al. Targeting insulin resistance in type 2 diabetes via immune modulation of cord blood-derived multipotent stem cells (CB-SCs) in stem cell educator therapy: phase I/II clinical trial. BMC Medicine. 11, 160 (2013).
- Delgado, E., et al. Modulation of autoimmune T-cell memory by stem cell educator therapy: phase 1/2 clinical trial. EBioMedicine. 2 (12), 2024-2036 (2015).
- Li, Y., et al. Hair regrowth in alopecia areata patients following Stem Cell Educator therapy BMC. Medizin. 13 (1), 87 (2015).
- Colombo, M., Raposo, G., Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell And Developmental Biology. 30, 255-289 (2014).
- Abak, A., Abhari, A., Rahimzadeh, S. Exosomes in cancer: small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. PeerJ. 6, 4763 (2018).
- Adamiak, M., Sahoo, S. Exosomes in myocardial repair: advances and challenges in the development of next-generation therapeutics. Molecular Therapy. 26 (7), 1635-1643 (2018).
- Akyurekli, C., et al. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Review and Report. 11 (1), 150-160 (2015).
- Hu, W., Song, X., Yu, H., Sun, J., Zhao, Y. Released exosomes contribute to the immune modulation of cord blood-derived stem cells (CB-SC). Frontiers in Immunology. (11), 165 (2020).
- Jacobson, G., Kårsnäs, P. Important parameters in semi-dry electrophoretic transfer. Electrophoresis. 11 (1), 46-52 (1990).
- Dykstra, M. J., Reuss, L. E. . Biological Electron Microscopy: Theory, Techniques, and Troubleshooting. , (2011).
- Konoshenko, M. Y., Lekchnov, E. A., Vlassov, A. V., Laktionov, P. P. Isolation of extracellular vesicles: general methodologies and latest trends. BioMed Research International. 2018, 8545347 (2018).
- Witwer, K. W., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. Journal of Extracellular Vesicles. 2, (2013).
- Orecchioni, M., Ghosheh, Y., Pramod, A. B., Ley, K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Frontiers in Immunology. 10, 1084 (2019).

