A New Workflow for Sampling and Digitizing Increment Cores
Summary
We present a protocol to use 3D-printed mounts to fix increment cores in the field without the necessity of unpacking and gluing them on wooden mounts. The new GSC holder allows placing the cores in a core microtome to cut their surface and directly transfer them to digital image capturing.
Abstract
Here we present a new workflow from taking increment cores in the field, storing and transporting them to the lab, to digitizing their tree rings for further analyses for subsequent dendroecological analyses. The procedure involves the use of new sample carriers for increment cores. These new Gärtner Schneider Core (GSC) holders are designed using three-dimensional (3D) modeling software and finally printed with a 3D printer. Using these mounts from the beginning in the field, the cores can directly be cut with a core microtome, and their surface can then be digitized without further rearrangement using a new high-resolution image-capturing system. They are thus available for direct analysis. This system allows digitizing tree rings from cores and disks, and also taking images from long micro sections (up to 40 cm) using transmitted light. This feature is of special interest for dendroecological and geomorphic applications to identify the onset of any disturbance in micro sections cut with a core-microtome.
Introduction
The principle of dating tree rings by applying the cross-dating technique was introduced the first time by the Austrian forest scientist Arthur Freiherr von Seckendorff-Gudent in 18811. In the first half of the 20th century, this technique was reinvented by the "Father of Dendrochronology" Andrew Ellicott Douglass, who intensively applied it in dating archaeological sites and living trees2.
Nowadays, dendroecology, the research topic acting as a kind of environmental frame of dendrochronology, is defined as the study of tree rings and their inherent growth variations caused by ecological and environmental changes in time3. In dendroecological research, many other characteristics than ring-width variations, such as stable isotopes, late wood density, or cell characteristics within single rings, are used to correlate these data to environmental parameters to better understand the impact of environmental conditions on tree growth over time4. Through the ongoing integration of wood anatomical studies to dendroecological research, dendroecology research evolved in the last decade and is more than ever a backbone in reconstructing past climate conditions5,6,7,8.
Although the technical development regarding sample preparation and analysis, especially in wood anatomy, was strong in the last decade9,10,11,12,13,14, there was almost no real advance regarding the simplification of sampling techniques15. Despite, e.g., acoustic wave technology16, until nowadays there is no reliable "non-destructive" method to extract the characteristics of rings from trees.
Consequently, all tree-ring-related studies still rely on wooden samples taken from trees or shrubs taken at the sites of interest. When focusing on trees, the standard procedure is taking increment cores from stems15.
Taking cores by using increment corers is frequently expressed as a "non-destructive" technique17. Compared to taking disks from stems, this is correct; nevertheless, this sampling technique causes a hole in the stem of about 1 cm in diameter, mostly reaching beyond the pith of the stem3. The tree is able to close this wound on its own, but this process causes growth reactions, altering the common structure in the close vicinity of the wound as well as a more or less intense discoloration of the existing wood around the hole because of fungal diseases18,19. So, it should better be called "minimally invasive" rather than "non-destructive".
The technique of taking increment cores evolved recently through the ability to use mechanical drills, resulting in higher quality samples, especially for wood anatomical analyses15. This procedure also saves a lot of time in the field compared to manual coring. What remained unchanged was the procedure of handling the cores, starting from the extraction from the tree to labeling, storing for transport, and preparing them in the lab for various possible analyzing techniques.
Cores still need to be packed in stable containers, such as straws made of plastics or paper, to prevent them from breakage during transport. Labeling the cores is done directly on the core using soft pencils or (more frequently) on the outside of each straw. When using plastic containers, the cores must be taken out after a short time to avoid the spread of fungi. So, the cores need to be taken out of the containers again. To stabilize the cores and to prevent them from bending when they start drying, the cores need to be fixed on a mount. This also helps with the subsequent surface preparation for further analyses. When doing so, the labels also need to be transferred to the respective mounts. A standard procedure is gluing the cores on wooden mounts or fixing them with tape in the rills of corrugated boards. Gluing them on wooden mounts is the most frequently used technique. Although this procedure is perfect for stabilizing and sanding or cutting the cores, it has several disadvantages regarding potential chemical, isotopic, and even wood anatomical analyses. Another disadvantage, despite the time required, is the error-prone transfer of the labels for each core to the new mounts.
In dendrochronology, ring-width measurements as a base for accurate dating are the backbone of all dendroecological studies20. Although many labs still rely on manual measurements using measurement tables, e.g., Lintab21 with attached binoculars, there is a trend of using flatbed scanners to digitize core surfaces and measure ring-width using software such as CooRecorder22 or WinDENDRO23. Unfortunately, these scanners, e.g., the widely used Epson Expression 10000XL do not have sufficient resolution to clearly depict structures as earlywood or latewood tracheids (Figure 1). For this reason, the resulting images are not suitable for recognizing difficult structures such as very narrow rings or density fluctuations, which are critical for an accurate cross-dating procedure without going back to the original cores using binoculars24,25.
Since high image resolution is an indispensable prerequisite for adequate image analyses in tree-ring science10, a new image-capturing system was developed at WSL (Skippy; https://www.wsl.ch/en/services-produkte/skippy/) to digitize tree rings on core surfaces using a digital camera resulting in images presenting a higher resolution than all existing flatbed scanners. This system was based on the idea of the ATRICS-system26, developed in 2007. Most recently, a simple but efficient image-capturing system comparable to the Skippy was presented as a self-assembly kit27.
Digitizing tree rings, i.e., reflected light image capturing, is an important step in creating high-resolution images of increment cores or disks to support a time-efficient, digitally based ring-width measurement. The system developed at WSL also allows taking images from long micro sections (up to 40 cm) using transmitted light. This additional feature is, for example, of interest for dendrogeomorphic applications to identify the onset of reaction wood in micro sections.
In the study, we present a protocol to ease the process of handling cores in the field and the lab. The base of the new technique presented is a reusable mount; the new GSC-holder GärtnerSchneiderCore (GSC) holder designed using 3D-modeling software and printed with a 3D printer. The GSC-holder allows for straightforward handling of the cores taken in the field without repacking or relabeling them. We also present an efficient new system for digitizing the prepared surfaces of the cores. This protocol spans the entire procedure from taking cores in the field to sample preparation, digitizing the core surfaces for subsequent analyses, and eventually storing them in an archive.
Protocol
1. Creating the GSC-holder
- Open the 3D model of the holder in a slicer program that is compatible with a 3D printer. Create a print file that can be read by the 3D printer (in this case, a "*.gcode" file).
NOTE: The 3D model can be designed using any 3D modeling software. - Transfer the print file to the 3D printer using a memory card or a USB stick and activate the print file on the 3D printer.
- As soon as the holder is printed, wait until the holder is cooled down to room temperature (RT). Then, remove the plate the holder sticks to from the printer and bend the plate a little until the form separates from the surface.
- Remove all excess threads or attachments from the holder.
NOTE: The number of holders to be printed at once depends on the size of the printer. On a 3D printer with a plate dimension of 36 cm x 36 cm, one can print around 30 holders with a length of 35 cm in one run. The time needed to print 30 holders depends on the device. On average, this should be done in approximately 8 h (overnight printing).
2. Extracting, stabilizing, and transporting increment cores in the field
- Take a cordless drill equipped with a torque booster and an increment corer, select the position of coring, and place the corer perpendicular to the growing axis of the stem.
NOTE: The same can be done manually using the increment corer without a cordless drill. - Start coring until the corer reaches at least half of the stem diameter. Check the depth as explained above by holding the extractor (which has the same length as the corer) alongside the corer.
- In the case of using a cordless drill, take off the drill, place the handle on the corer (which is already the case when using the increment corer manually), take the extractor with the open side facing upwards, and insert it fully into the corer.
- Turn the increment corer backward (one full turn) to break the core off the stem. Take out the extractor, including the core.
- Remove the core from the extractor. Check the fiber direction of the core to ensure an upright orientation of the fibers when placing the core in the holder.
NOTE: Fiber direction can be checked at both ends of the core as well as on the side of the core. For this, hold the core against the light and turn it until a side shining is seen. This happens because, on this side, the radial cell walls are cut longitudinally and reflect light differently than the rest of the core. - Place the core on top of the holder with the fiber direction upright. Press on top of the core with all fingers until the core slides into the holder.
- Label the core on the side of the holder using a soft pencil, enabling writing even on glass.
NOTE: The writing can later be removed with a common rubber. - Place the holder with the core into the transport box and close the cover.
3. Preparing the mounted cores in the lab
- OPTIONAL: Embedding the mounted cores in paraffin for potential micro sectioning.
- Place a steel box with a lid fitted with a valve for a vacuum pump connection on a hotplate, fill it up to approximately 2 cm with paraffin, and wait until it has completely melted.
- Take the mounted cores out of the transport box. Place the holders with the cores as they are in the liquid paraffin and close the lid.
- Start the vacuum pump, apply a constant, light vacuum to the container, and wait for about 2 h. Due to the open structure of the holder, the paraffin can penetrate the cores without additional barriers.
- Stop the vacuum pump and open the lid. Take out the holders with the cores, place them on a grid, and let them cool down.
- If needed, remove surplus paraffin from the sides of the holder.
- Preparing the core surfaces
- Take the mounted cores out of the transport box or the paraffin bath. Place the holder with the core as it is in the sample holder of a core-microtome. Make sure to orient the core in a way that the latewood of the rings faces toward the blade.
- Tighten the screws of the sample holder until the core holder is completely secure.
- Lift the sample holder until the core slightly touches the blade. Pull the blade over the entire extent of the core to cut off the first part of the top.
- Push back the knife behind the core, lift the sample holder a few microns, and repeat the procedure until a plane surface of at least 2-3 mm in width is obtained.
- As soon as the surface is cut as intended, remove the core holder from the sample holder of the microtome.
NOTE: Cutting the cores with a core-microtome and not sanding them is recommended because the surface is cleaner and straight, and the cells are not filled with dust.
4. Digitizing the core surfaces
- Place the core holder with the plain core surface on the table of an image-capturing system, as the WSL-Skippy system is presented here.
- Make sure to align the core holder with the moving direction of the table or camera.
- Position the table with the core holder below the camera to have the outermost ring in the center of view below the camera objective.
- Place a scale next to the onset of the core and take an image for calibration purposes.
NOTE: This only needs to be done once when doing images of many cores in succession. - Define the length of the core in the software and start the image-capturing process. When the last image is taken, the table moves back to the starting position.
- Remove the sample from the table, place the next holder below the camera, and repeat the procedure described before by defining the length of the core until all cores are photographed.
- Use a (distortion-free) stitching software, e.g., PTGui, to combine the single images into one final image of the core surface.
5. Storing the cores
- Take the analyzed cores in the holder and place them in the portable storage rack printed with a 3D printer.
- Label the rack to identify the cores from the outside.
- Store the rack on a shelf or any other archive available.
Representative Results
GSC-holder
The core holders are (as a default) printed to a length of 35 cm, which corresponds almost to the maximum print size of the 3D printer used (Original Prusa XL, build volume 36 cm × 36 cm × 36 cm). In case longer cores are taken, the holder can be extended with additional holders by connecting them with small connecting pieces through the indentations that are present on both ends of all holders (Figure 2A).
When doing fieldwork, the time needed to store the cores directly in the holder is comparable to simply putting them into a straw or other wrapping material. Although there is a need to respect the fiber direction of each core before pressing the core into the holder (Figure 2), this additional time is only a few seconds and can be supported by using a lens. In our experience, the extra time needed (if any) sums up to about 1 min for 10 cores. This minimal extra time also refers also to broken cores. Instead of taking broken cores piece by piece into a straw, these pieces are simply placed in the holder one after the other and pressed in.
To guarantee the safe transport of the cores in the field and to the lab, we designed and printed a special transport box for the holders, including the cores (Figure 3). The holders can simply be placed in the box where they are stabilized by little bulges that fit exactly into the indentations on both ends of the holders. The box can then be closed by a cover that is pushed in at the side grooves of the box.
The real advantage of the new holder becomes apparent in the laboratory. Instead of taking the cores out of the straw (or other containers), preparing wooden mounts with clue, fixing the cores on the mount, transferring the label to the new mount and waiting at least a few hours until the glue is dry and stable for further processing, the cores in the holder can directly (i) be fixed in a core microtome to cut a plain surface or (ii) can be sanded directly with a sanding machine without any further preparational process needed. The elimination of the need to transfer the labels to any new mounts especially avoids possible transmission errors.
Since the holders can be designed for any core diameter, it does not matter if they are needed for "standard" 5 mm cores or 10 mm or 12 mm cores as they are taken for, e.g., isotope or other chemical analyses.
Related to isotope or chemical analyses, the advantage of the holder is that the cores are fixed without the need for glue or a fixation medium. So, the cores are not contaminated, and they can easily be removed from the holder for more specific analyses. Also, regarding wood anatomical analyses, the ability to easily remove the cores from the holder enables straightforward handling of the cores for preparing micro sections.
The optional possibility of embedding cores fixed in the holder allows for stabilizing sensitive structures, as cells with thin cell walls tend to break while cutting. Stabilizing the core by embedding it in paraffin is in many cases, more efficient than simply adding a cornstarch solution.
Another advantage also appears when the cores need to be stored for any later inspections or re-analyzes. The holders can be placed in specially designed racks (Figure 4) and also printed using a 3D printer comparable to the storage in the transporting box. The GSC-holders with the cores are placed in the rack as they are and can then be stored at any place. The width of the racks, i.e., how many cores can be fixed in a single rack depends on the space available on a shelf or storage room. The rack models can be adapted to any specific need and printed accordingly.
Digitizing core or disk surfaces
The image capturing system, developed at WSL (Figure 5), allows digitizing tree rings (automated image capturing) to create high-resolution images of increment cores or disks to support a time-efficient, digitally based ring-width measurement. The system consists of a plate fixed on a threaded rod moving the sample below the objective (Sony FE 90 mm f/2.8 Macro) of a 61 MP camera (Sony Alpha 7R IV) in predefined steps between 0.1 to 1 cm. The images are taken using the autofocus system of the camera to guarantee focused single images. The resolution of the camera allows a real resolution of each image of 6500 dpi tested using a SilverFast resolution target (USAF 1951). This might sound a little low compared to the official resolution of a flatbed scanner with a specified resolution of 4800 dpi. But when testing images of the same target taken with an Epson XL scanner using the 4800 dpi resolution, the resulting images showed a real resolution of 1825 dpi only (Figure 6). The high resolution of the images allows a clear view of the single cells and for this, a clear definition of the ring boundaries captured in the images (Figure 7). If the surface of the cores or the disks used is well prepared, there is no need to go back to the original sample to check the structure again. After stitching the single images, the resulting core images can be analyzed using the preferred analyzing software.
The image capturing system also allows taking images from micro sections up to a length of 40 cm using transmitted light. This feature is of interest for, e.g., dendrogeomorphic applications to identify the onset of reaction wood or other specific features in micro sections of entire tree cores (Figure 8).

Figure 1: Scan images of Larix decidua Mill. Tree rings scanned at different resolutions using a flatbed scanner. Please click here to view a larger version of this figure.
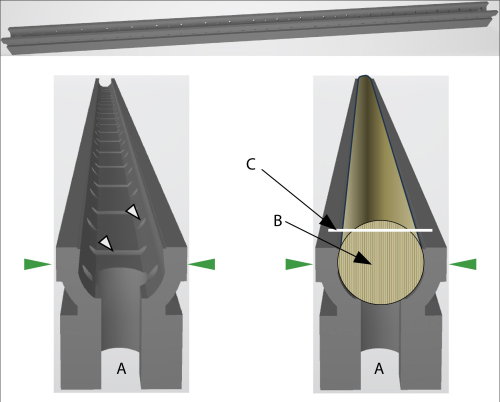
Figure 2: Schematic view of the GSC-holder. (A) The opening at both ends of the holder allows connecting two holders with a little pole to fix longer cores. The green arrows indicate the pressure direction when the holder is fixed in the core microtome. Left: White arrows indicate openings that allow air or liquid (for embedding) circulation. Right: The GSC-holder with a core pressed in. (B) The fiber direction of the core needs to be upright. (C) The white line indicates the cutting surface of the core. Please click here to view a larger version of this figure.

Figure 3: Transporting box to store and transport the GSC-holder with the cores in the field. Please click here to view a larger version of this figure.
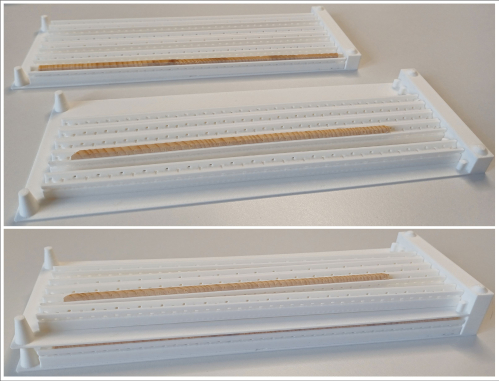
Figure 4: Storage frames to place the GSC-holders in for final storage in an archive. The frames can be stacked to save space. Please click here to view a larger version of this figure.
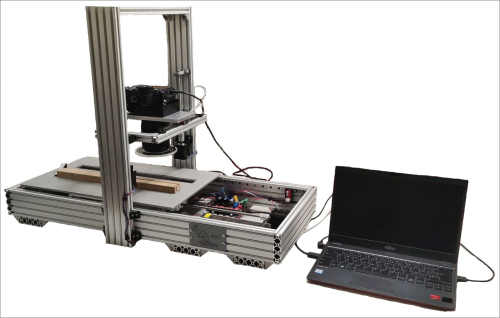
Figure 5: The image-capturing system developed at WSL. Please click here to view a larger version of this figure.

Figure 6: Comparison of image resolution between a flatbed scanner and the image capturing system. (A) SilverFast resolution-target (USAF 1951). (B) Image scanned with a flatbed scanner with a specified resolution of 4800 dpi (interpolated) and respective section enlargements below. (C) Image taken with the image capturing system and respective section enlargements below. Please click here to view a larger version of this figure.

Figure 7: Composite image of a Larix decidua Mill. increment core (top image) and respective section enlargements below. Single images of the composite were taken with the image-capturing system. Please click here to view a larger version of this figure.
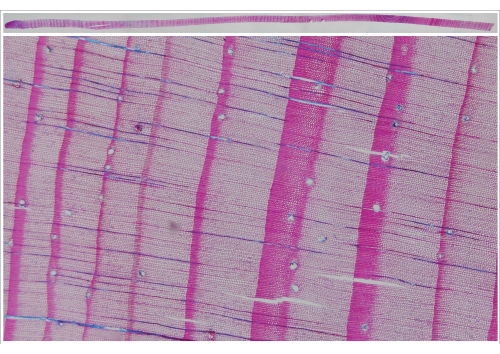
Figure 8: Composite image of a micro section of an entire increment core (Larix decidua Mill.) and respective section enlargement. Single images of the composite were taken the image-capturing system (transmitted light). Please click here to view a larger version of this figure.
Discussion
The inclusion of wood anatomy in dendroecological studies widely opened these studies for new and in-depth analyses of past environmental conditions28,29,30. These new techniques also intensified the analytical efforts, i.e., lab time needed to generate the data of interest. There have been numerous attempts to optimize lab work and to reduce the time needed in the lab regarding wood anatomical techniques9,12,13,15,30. But almost no efforts have been taken to ease the common procedure of handling, preparing, and storing cores taken for these studies.
3D printing offers new possibilities in this regard9. The new, 3D-printed core holder is the first attempt to simplify this whole procedure, making it less time-consuming and, for this, more efficient.
While cores stored in plastic straws31,32 or comparable containers need to be taken out to prevent fungi from developing on the outside (and soon also the inside) of the core, cores fixed in the GSC-holders can stay as they are. To this point, it is comparable to storing them in paper straws33.
The advantage becomes clear as soon as the whole procedure of (i) removing the cores from the straw (or other container), (ii) gluing them on wooden mounts or fixing them on other objects as cable supports, and (iii) the possibly error-prone process of transferring the respective code used for each core as it was almost a standard for decades now34, becomes unnecessary.
The open structure of the GSC-holder allows storing the cores without the risk of fungal infestation, as it would be the case when stored in a plastic container. As described above, the holder also allows an embedding in paraffin to stabilize the structure. Nevertheless, this "simple" embedding cannot be compared to common embedding procedures using cassettes to embed the sample in a Paraffin block as it is done for micro cores35. The simple technique is rather comparable to applying corn starch when cutting micro sections36. It will better stabilize the cells and prevent them from breakage during the cutting procedure, but it is more time-consuming than simply adding corn starch. This form of embedding will not stabilize the whole core as if it were embedded in a block. If the core is broken, the sections will also break. Since the GSC-holder fits in the core-microtome37, preparing the surface for the subsequent digitizing process only takes a few minutes.
For the process of digitizing tree rings the application of flatbed scanners, frequently used for blue intensity measurements38,39, was not satisfactory regarding more detailed views of the ring structure because of the rather low quality of the resulting images. Although the boundaries of common (wide) rings of conifers were visible in these images, narrow rings, or even density fluctuations, were nearly impossible to identify.
Although there are fascinating new attempts to digitize tree rings in high resolution, such as x-ray CT40, using digital cameras with high resolution is still the most efficient and cost-effective way to produce high-quality images for further measurements.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors wish to thank Prof. Jussi Grießinger for supporting the idea of creating the new holder.
Materials
| Core-microtome | WSL | https://www.wsl.ch/en/services-produkte/microtomes/ | Microtome to cut micro sections from increment cores |
| Epson Expression 10000XL | EPSON | https://epson.com/Support/Scanners/Expression-Series/Epson-Expression-10000XL—Graphic-Arts/s/SPT_E10000XL-GA | flatbed scanner |
| GSC holder | WSL | in-house | 3D printed mount to fix cores for transport, preparation, analyses, and storage |
| Skippy image capturing system | WSL | https://www.wsl.ch/en/services-produkte/skippy/) | Image capturing system developed at WSL equiped with a 61 MP camera (Sony Alpha 7R IV and Sony FE 90mm f/2.8 Macro lens) |
References
- Wimmer, R. Arthur Freiherr von Seckendorff-Gudent and the early history of tree-ring crossdating. Dendrochronologia. 19 (1), 153-158 (2001).
- McGraw, D. J. Andrew Ellicott Douglass and the giant sequoias in the founding of dendrochronology. Tree-Ring Res. 59 (1), 21-27 (2003).
- Schweingruber, F. H. . Tree Rings and Environment: Dendroecology. , (1996).
- Amoroso, M. M., Daniels, L. D., Baker, P. J., Camarero, J. J. . Dendroecology: Tree-Ring Analyses Applied to Ecological Studies (Vol.231). 231, (2017).
- Lopez-Saez, J., Corona, C., Von Arx, G., Fonti, P., Slamova, L., Stoffel, M. Tree-ring anatomy of Pinus cembra trees opens new avenues for climate reconstructions in the European Alps. Sci Total Environ. 855, 158605 (2023).
- Björklund, J., et al. Fennoscandian tree-ring anatomy shows a warmer modern than medieval climate. Nature. 620 (7972), 97-103 (2023).
- Camarero, J. J., Colangelo, M., Rodriguez-Gonzalez, P. M. Tree growth, wood anatomy and carbon and oxygen isotopes responses to drought in Mediterranean riparian forests. Forest Ecol Manag. 529, 120710 (2023).
- Huang, R., Xu, C., Grießinger, J., Feng, X., Zhu, H., Bräuning, A. Rising utilization of stable isotopes in tree rings for climate change and forest ecology. JForestry Res. 35, 13 (2024).
- Schneider, L., Gärtner, H. Additive manufacturing for lab applications in environmental sciences: pushing the boundaries of rapid prototyping. Dendrochronologia. 76, 126015 (2022).
- Björklund, J., et al. Scientific merits and analytical challenges of tree-ring densitometry. Rev Geophys. 57, 1224-1264 (2019).
- Katzenmaier, M., Garnot, V. S. F., Björklund, J., Schneider, L., Wegner, J. D., von Arx, G. Towards ROXAS AI: Deep learning for faster and more accurate conifer cell analysis. Dendrochronologia. 81, 126126 (2023).
- Gärtner, H., Lucchinetti, S., Schweingruber, F. H. A new sledge microtome to combine wood anatomy and tree-ring ecology. IAWA J. 36 (4), 452-459 (2015).
- Gärtner, H., et al. A technical perspective in modern tree-ring research – how to overcome dendroecological and wood anatomical challenges. J Vis Exp. 97 (e52337), (2015).
- Gärtner, H., Banzer, L., Schneider, L., Schweingruber, F. H., Bast, A. Preparing micro sections of entire (dry) conifer increment cores for wood anatomical time-series analyses. Dendrochronologia. 34, 19-23 (2015).
- Gärtner, H., Schneider, L., Lucchinetti, S., Cherubini, P. Advanced workflow for taking high-quality increment cores – new techniques and devices. J Vis Exp. (193), e64747 (2023).
- Wang, X. Acoustic measurements on trees and logs: a review and analysis. Wood Sci Technol. 47, 965-975 (2013).
- Steenkamp, C. J., Van Rooyen, M. W., Van Rooyen, N. A non-destructive sampling method for dendrochronology in hardwood species. South Afr For J. 186, 5-7 (1999).
- Toole, E. R., Gammage, J. L. Damage from increment borings in bottomland hardwoods. J For. 57, 909-911 (1959).
- Grissino-Mayer, H. D. A manual and tutorial for the proper use of an increment borer. Tree-Ring Res. 59 (2), 63-79 (2003).
- Griffin, D., et al. Gigapixel macro photography of tree rings. Tree-Ring Res. 77, 86-94 (2021).
- . LINTAB-Precision ring by ring Available from: https://rinntech.info/products/lintab/ (2003)
- . Regent Instruments Available from: https://regentinstruments.com (2024)
- De Micco, V., et al. Intra-annual density fluctuations in tree rings: How, when, where, and why. IAWA J. 37, 232-259 (2016).
- Edwards, J., et al. Intra-annual climate anomalies in northwestern North America following the 1783-1784 CE Laki eruption. J Geophys Res Atmos. 126, e2020JD033544 (2020).
- Levanič, T. ATRICS-A new system for image acquisition in dendrochronology. Tree-Ring Res. 63 (2), 117-122 (2007).
- García-Hidalgo, M., et al. CaptuRING: A do-it-yourself tool for wood sample digitization. Methods Ecol Evol. 13 (6), 1185-1191 (2022).
- Rodriguez, D. R. O., et al. Exploring wood anatomy, density and chemistry profiles to understand the tree-ring formation in Amazonian tree species. Dendrochronologia. 71, 125915 (2022).
- Gärtner, H., Farahat, E. Cambial activity of Moringa peregrina (Forssk.) Fiori in arid environments. Front Plant Sci. 12, 760002 (2021).
- Gärtner, H., Lucchinetti, S., Schweingruber, F. H. New perspectives for wood anatomical analysis in dendrosciences: the GSL1-microtome. Dendrochronologia. 32, 47-51 (2014).
- Maeglin, R. R. . Increment Cores: How to Collect, Handle, and Use Them (Vol. 25). , (1979).
- Agee, J. K., Huff, M. H. . The Care and Feeding of Increment Borers. , (1986).
- Phipps, R. L., , . . Collecting, Preparing, Crossdating, and Measuring Tree Increment Cores (No. 85-4148). , (1985).
- Cole, D. M. . Protection and Storing Increment Cores in Plastic Straws. 216, (1977).
- Rossi, S., Anfodillo, T., Menardi, R. Trephor: a new tool for sampling microcores from tree stems. IAWA J. 27 (1), 89-97 (2006).
- Schneider, L., Gärtner, H. The advantage of using a starch based non-Newtonian fluid to prepare micro sections. Dendrochronologia. 31, 175-178 (2013).
- Gärtner, H., Nievergelt, D. The core-microtome: A new tool for surface preparation on corse and time series analysis of varying cell parameters. Dendrochronologia. 28 (2), 85-92 (2010).
- McCarroll, D., Pettigrew, E., Luckman, A., Guibal, F., Edouard, J. L. Blue reflectance provides a surrogate for latewood density of high-latitude pine tree rings. Arct Antarct Alp Res. 34 (4), 450-453 (2002).
- Björklund, J., Seftigen, K., Kaczka, R. J., Rydval, M., Wilson, R. A standard definition and terminology for Blue Intensity from conifers. Dendrochronologia. 85, 126200 (2024).
- Van den Bulcke, J., et al. Advanced X-ray CT scanning can boost tree ring research for earth system sciences. Ann Bot. 124 (5), 837-847 (2019).

.
