Molecular Imaging of Human Brain Organoids Using Mass Spectrometry
Summary
An advanced method was developed for mass spectrometry imaging (MSI) of brain organoids that allows mapping metabolite distributions within these models. This technology offers insights into brain metabolic pathways and metabolite signatures during early development and in disease, promising a deeper understanding of the human brain function.
Abstract
Brain organoid models serve as a powerful tool for studying human brain development and function. Mass spectrometry imaging (MSI), a cutting-edge technology, allows us to map the spatial distribution of diverse molecules such as lipids, sugars, amino acids, drugs, and their metabolites within these organoids, all without the need for specific molecular probes. High-quality MSI data hinge on meticulous sample preparation. Fixatives play a pivotal role, but conventional options such as glutaraldehyde, paraformaldehyde, and cryopreserving such as sucrose may inadvertently impact tissue metabolites. Optimal fixation entails flash freezing in liquid nitrogen. However, for small organoids, a more suitable approach involves transitioning the organoids directly from the incubator into a warmed embedding solution, followed by freezing in dry ice-cooled ethanol. Another critical step is the embedding prior to cryosectioning, which also requires materials compatible with MSI, as traditional options can interfere with matrix deposition and ionization. Here, an optimized protocol for high resolution-MALDI-MSI of human brain organoids is presented, encompassing sample preparation, sectioning, and imaging using mass spectrometry. This method showcases the molecular distribution of small metabolites, such as amino acids, with high mass accuracy and sensitivity. As such, coupled with complementary studies of brain organoids, it can assist in illuminating complex processes governing early brain development, metabolic cell fate trajectories, and distinctive metabolite signatures. Furthermore, it provides insights into the precise locations of molecules within the organoid, enriching our understanding of the spatial organization of 3D brain organoid models. As the field continues to advance, a growing number of studies leveraging MSI to delve into brain organoids and complex biological systems is anticipated, thereby deepening the understanding of the metabolic aspects of human brain function and development.
Introduction
Organoid models, derived from primary tissue stem cells, embryonic stem cells, or induced pluripotent stem cells (iPSCs)1,2,3 have advanced human biology research by offering three-dimensional models that closely mimic organ-specific functions, aiding in the study of human development, disease mechanisms, and drug discovery4,5. Within this context, unraveling brain organoid complexities is pivotal for understanding both physiological and pathological brain development6,7, necessitating technologies such as mass spectrometry imaging (MSI)8,9. MSI, distinct from traditional mass spectrometry, enables direct, label-free mapping of hundreds to thousands of biomolecules within a single tissue section, providing detailed insights into the spatial distribution of molecules-like lipids, peptides, amino acids, drugs, and their metabolites-without the need for specific molecular probes10,11. Moreover, MSI molecular images can be co-registered onto histological and immunostained sections, providing a comprehensive view of tissue morphology, cell specificity, and molecular content.
The MSI holds significant promise for organoid research, offering insights into the molecular basis of diseases, genetic-phenotype relationships, and responses to environmental stimuli12,13,14,15. In the pharmaceutical industry, MSI facilitates the analyses of drug absorption, distribution, metabolism, and elimination in preclinical models11,16. Furthermore, it aids in resolving their bio-transformed metabolites, which may be pharmacologically active17.
Among MSI methods, matrix-assisted laser desorption/ionization (MALDI), desorption electrospray ionization (DESI), and secondary ion mass spectrometry (SIMS) predominate9,18,19,20,21. Of these, MALDI-MSI stands out for its versatility, wide mass range, direct analysis capabilities, and compatibility with various tissue-specific chemical compounds22. However, despite its potential, MALDI-MSI's application in brain organoid research remains underexplored. To address this gap, a tailored protocol for high-resolution MALDI-MSI (HR-MALDI-MSI) analysis of brain organoids has been introduced to optimize tissue preservation, matrix selection, and imaging conditions, ensuring reliable acquisition of high-quality data15. This detailed protocol showcases the capabilities of HR-MALDI-MSI to provide researchers with the additional arsenal to harness the power of this technology to explore the metabolic landscape of organoids in unprecedented detail.
Protocol
The general overflow of the protocol is depicted in Figure 1. The details of the reagents and the equipment used in the study are listed in the Table of Materials.
1. Brain organoid section preparation
- Prepare brain organoids (2-3 mm in size when they reach 30-60 days in culture) from induced pluripotent stem cells (iPSC) following previously published reports23,24 and collect them at a desired time using wide bore tips.
- Wash the prepared organoids three times with 1x Dulbecco's phosphate-buffered saline (DPBS) without CaCl2 and MgCl2 at room temperature to rinse the media and then very quickly with distilled water at room temperature to rinse any salts from the DPBS.
- Prepare 10% of gelatin solution from cold fish skin by stirring and heating the gelatin (10 mg in 100 mL of DPBS) at 70-80 °C for 2 h, then move the solution to 37 °C incubator for 30 min to remove bubbles and equilibrate to the temperature of the incubator where organoids were grown.
NOTE: It is recommended to prepare gelatin fresh for each experiment, but it can be used for up to 1 week (store at 4 °C) if needed. Gelatin will solidify at room temperature and needs to be warmed up prior to use as an embedding solution in order to become liquid. - Pin down the organoid in the center of a plastic mold (25 mm x 20 mm x 5 mm) with the small pipette tip and gently pour the 10% gelatin embedding solution into the mold until the organoid is fully immersed (mold should be approximately half-filled).
- Place the mold into a Petri dish on dry ice containing cold 100% ethanol to rapidly freeze it (1-2 min). When completely frozen, evidenced by the change of color to solid white, take out the organoid-gelatin block from the Petri dish, wrap it in aluminum foil or place it in a tin cup, sealed to prevent water concentration, and store at −80°C until ready to cryosection.
- For cryosectioning, place the plastic blocks containing organoids within the cryo-chamber at -20 °C to -25 °C for 10-15 min to equilibrate with the chamber temperature. Section the organoids into 14 µm sections using cryotome and thaw-mount on indium tin oxide-coated glass slides (ITO slides) for MSI.
- Either immediately scan structurally uniform sections in the mass spectrometer or seal them in the container and store them at -80°C for later studies.
2. Matrix preparation and application for MALDI-MSI
- For MALDI-MSI, spray the slides and coat the tissue sections with the matrix, an organic compound that helps ionize different metabolites25. Different matrices are used for imaging different species of molecules; therefore, first, the most appropriate matrix for the study must be chosen.
NOTE: This study focused on the localization and distribution of small metabolites and amino acids related to mitochondrial metabolic pathways, and the matrix was chosen accordingly (for mapping lipids, please see Cappuccio et al.)15. - First, upon taking out the ITO slides from -80 °C, put the slide immediately in a desiccator for 20 min to minimize condensation of atmospheric water on the surfaces.
- Prepare N-(1-naphthyl) ethylenediamine dihydrochloride (NEDC), which is a suitable matrix for small metabolites as it gives a strong signal of the analyte of interest without interfering with background signals under the m/z of 500 Da26. Spray 10 mg/mL NEDC solution in 70% methanol onto the organoid sections after slide desiccation using a heated pneumatic sprayer.
- Set the matrix sprayer parameters as follows: nozzle temperature = 75 °C, nozzle velocity = 1,250 mm/min, pump flow rate = 100 µL/min, number of passes = 8, track spacing = 2.5 mm, gas flow rate 3L/min, drying time =10 s and 10 psi nitrogen gas pressure.
3. MALDI-MSI instrumentation
- To achieve a high-resolution MALDI-MSI platform for visualizing brain organoid metabolites, mount a MALDI ion source with a dual-ion funnel interface to a mass spectrometer.
- Use a connected Q-switched, frequency-tripled Nd laser with a 349 nm wavelength, a repetition rate of 1 kHz, and a pulse energy of approximately 1.3-1.4 µJ.
- To avoid oversampling, focus the laser to a spot size of ~15 µm in diameter.
- Attach the sample to the MALDI injector stage. Operate and maintain the high-pressure ion funnel at 7.4-7.5 Torr, and keep the low-pressure ion funnel at 1.6-1.8 Torr.
- Apply radio frequency voltages of 604 kHz, 80 V0-peak, and 780 kHz, 191 V0-peak to the high- and low-pressure ion funnels, respectively.
- To improve the sensitivity for small metabolites in the low mass range, reduce the RF amplitudes in the low and high-pressure funnel of the MALDI-MSI source to approximately 20% and 15%, respectively.
- Set the mass resolution to 70,000. Select the area and the pixel size (25 µm/pixel) to be measured in MALDI injector software.
4. MALDI-MSI data acquisition and data analysis
- Acquire data in the m/z range of 80-900 in both negative and positive ion modes. Scan the samples with a lateral resolution of 25 µm per pixel for 14 µm organoid sections.
- Set the ion injection time on the mass spectrometer at 250 ms and acquire Fourier Transform Mass Spectra (FTMS) in the profile mode while the automatic gain control (AGC) is turned off26.
- Use the NEDC matrix peaks as an internal online mass calibration, resulting in a mass accuracy of better than ±5 ppm.
- Use compatible software to process the data. Import the MSI spectral data directly into the software, then perform baseline correction using a convolution algorithm and normalize the data using total ion count (TIC).
- Generate the feature list of ion images from the raw data files using a bin width of Δm/z = 0.01 or ±5 ppm to distinguish m/z images based on mass defect and pixel coverage.
- Generate false color (Jet) or RGB (red-green and blue) images from individual metabolite ion species. Upload the m/z list of raw data files obtained from MALDI-MSI to the Human Metabolome Database (HMDB) (mass tolerance <5 ppm relative to the theoretical m/z) to identify metabolites.
NOTE: The exact mass and predicted formula and structure of the metabolite obtained from the LC-MS/MS data are also used to query metabolomics databases (e.g., HMDB, Metlin) for metabolite comparison and identification.
Representative Results
Here is a protocol that optimizes molecular (metabolic) imaging using MSI (Figure 1, please also see Cappuccio et al.)15. The most reliable data and tissue morphology preservation were achieved with 10% gelatin from cold fish skin, as confirmed by histological staining of serial sections. Using fish gelatin embedding of 60-day human brain organoids, the Krebs cycle-related metabolites were mapped using MSI, demonstrating their spatial distribution (Figure 2).
Overall, the optimized protocol consistently delivered robust results, with 10% gelatin from cold fish skin as the preferred embedding material and NEDC matrix spray settings fine-tuned to enhance image resolution. The detected small metabolites in brain organoids provide valuable insights into the molecular complexities of this tissue.
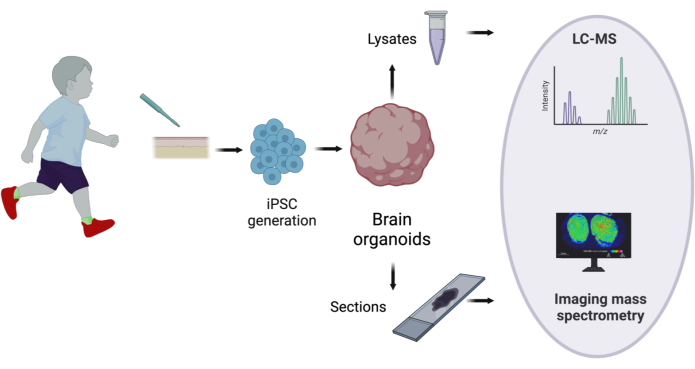
Figure 1: General pipeline of human brain organoid metabolome study. Organoids derived from individual's fibroblasts via induced pluripotent stem cells are used for LC-MS and MSI to highlight the spatial distribution of metabolites. Please click here to view a larger version of this figure.
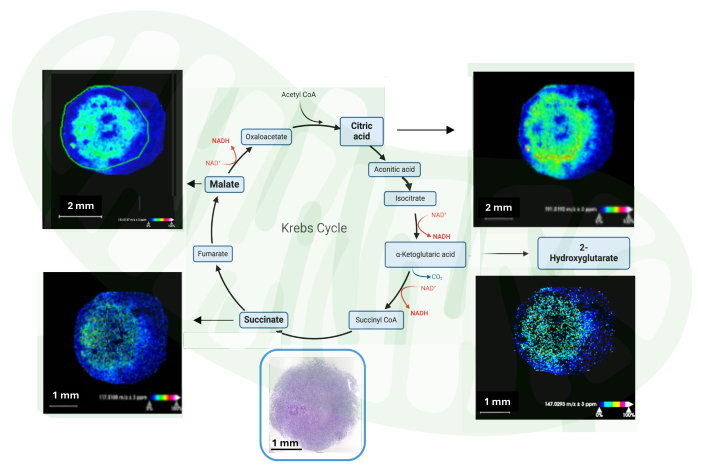
Figure 2: Mapping Krebs cycle-related metabolites. Identification and distribution of Krebs cycle-related metabolites using 60-day organoids derived from healthy controls using MSI. Hematoxylin and eosin staining are shown. Please click here to view a larger version of this figure.
Discussion
The refined protocol for high-resolution MALDI-MSI of human brain organoids meticulously addresses pivotal steps to ensure the reliability of results. Sample preservation emerges as paramount, and to circumvent tissue cracking and damage, an alternative freezing method is proposed involving a warmed embedding solution and dry ice-cooled ethanol. This protocol works best for tissue of the minute size, such as human brain organoids15. Embedding materials such as optimal cutting temperature (OCT) compound and Formalin-Fixed Paraffin Embedding (FFPE) are cautioned against due to their interference with matrix deposition and ionization. Instead, 10% gelatin from cold fish skin is highlighted as the optimal embedding solution, showcasing its efficacy in preserving tissue integrity during cryo-sectioning15. Further, the choice of matrix and its deposition techniques, such as dry spraying, is imperative to minimize the delocalization of low m/z small metabolites and enhance image resolution.
The robustness and reliability of the protocol are substantiated by the successful detection and visualization of a broad spectrum of molecules in human brain organoids15, yielding invaluable insights into their spatial distribution and potential biological significance. These findings significantly augment the comprehension of the intricate molecular landscape within brain organoids, elucidating pivotal signaling pathways and metabolic processes underpinning brain development and function.
While the current data provide new insights into the localization of diverse species, the Spectroglyph MALDI imaging source used in this study has yet to attain single-cell level resolution (currently at ~10-20 µm). Other platforms, such as Bruker's timsTOF and Water's MRT, can deliver single-cell resolution at 5 µm, and thus, it is important to keep in mind the instrument to be used for experimentation.
Despite these constraints, high-resolution HR-MALDI-MSI of brain organoids harbors considerable promise. The capability to map metabolite and lipid distributions within organoids offers a unique point on metabolic cell fate trajectories, pathways, and distinct signatures crucial for organoid development and maturation. This methodology serves as a bridge between conventional histology and molecular analysis, facilitating a deeper comprehension of the interconnections between genome, phenome, and environmental responses.
In summary, the optimized protocol for HR-MALDI-MSI of human brain organoids constitutes a valuable asset for researchers exploring organoid models. This method lays the groundwork for further elucidation of the molecular intricacies underpinning brain development and function by meticulously addressing critical steps, troubleshooting, and acknowledging limitations. As MSI technology evolves and becomes increasingly accessible, it is poised to advance our understanding of early human development, disease modeling, and drug discovery.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank members of the Maletic-Savatic laboratory, especially Danielle Mendonca, a graduate student, for helpful discussions and comments on this work, and Baylor College of Medicine Advanced Technology Core support of the NMR and Drug Metabolism Core. This work was supported in part by grants from the National Institute of Mental Health (1R01MH130356 to M.M.S), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R61/R33HD099995 to F. L.), the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (P50HD103555) for use of the Microscopy Core facility, the Pathology Core facility, and the Human Disease Cellular Models Core facility. In addition, this work has been funded in part with Federal funds from the Translational Research Institute for Space Health through NASA Cooperative Agreement NNX16AO69A, grant RAD01013 (M.M.S), NASA under contract 80ARC023CA004 titled ”MORPH: Multi-Organ Repair Post Hypoxia” (M.M.S.) as well as Autism Speaks (G.C), Simons Foundation Pilot Award (M.M.S.) and Cynthia and Antony Petrello
Endowment (M.M.S).
Materials
| (1S, 2S)-1,2-di-1-Naphthyl-ethylenediamine dihydrochloride | Sigma-Aldrich (St. Louis, MIA) | 1052707-27-3 | Matrix substance for MALDI-MS, ≥99.0% (HPLC) |
| 1× Dulbecco’s phosphate-buffered saline (DPBS) | Life Technologies, USA | 14190-144 | Without CaCl2 and MgCl2 |
| ART Wide Bore Filtered Pipette Tips | Thermo Fisher Scientific, Massachusetts, USA | 2069G | Reduce potential contamination |
| Cryomolds | Tissue-Tek | 25608-916 | Standard mold with flat-surface |
| Entellan | Fisher Scientific | M1079610500 | Cover slips |
| Eosin Y | Fisher Scientific (Waltham, MA, USA) | E511-25 | Certified Biological Stain |
| Fish gelatin | Sigma-Aldrich (St. Louis, MIA) | G7041 | Powder form from cold water fish skin |
| Hematoxylin | Fisher Scientific (Waltham, MA, USA) | 517-28-2 | Certified biological stain Elevated pressure imaging source with |
| HTX M5+ sprayer | HTX Technologies LLC, Carrboro, USA | Matrix sprayer | |
| Indium tin | Hudson Surface Technology, | PL-IF-000010-P25 | Provide a conductive surface for MALDI imaging |
| MALDI ion source | Spectroglyph LLC, USA | dual ion funnel interface | |
| Methanol | Fisher Scientific (Waltham, MA, USA) | 67-56-1 | HPLC grade |
| oxide (ITO) conductive–coated slides | New York, United States | ||
| Porcine gelatin | Sigma-Aldrich (St. Louis, MIA) | G1890 | Powder form from porcine skin |
| Q-Exactive mass spectrometer | Thermo Fisher Scientific, Massachusetts, USA | High resolution mass spectrometry | |
| SCiLS Lab software | version 2024a Pro | for processing the data | |
| Water | Fisher Scientific (Waltham, MA, USA) | W6500 | HPLC grade |
| (Waltham, MA, USA) |
References
- Sasai, Y. Next-generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell. 12 (5), 520-530 (2013).
- Clevers, H. Modeling development and disease with organoids. Cell. 165 (7), 1586-1597 (2016).
- Takebe, T., Wells, J. M. Organoids by design. Science. 364 (6444), 956-959 (2019).
- Sandoval, S. O., et al. Rigor and reproducibility in human brain organoid research: Where we are and where we need to go. Stem Cell Rep. 19 (6), 796-816 (2024).
- Dei-Ampeh, A. K., Shah, M., Cappuccio, G., Young, D. W., Maletic-Savatic, M. Human models as new tools for drug development and precision medicine. Phenotyping of Human iPSC-derived Neurons. , 155-171 (2023).
- Choi, W. T., et al. Metabolomics of mammalian brain reveals regional differences. BMC Sys Bio. 12 (S8), 127 (2018).
- Kandel, P., et al. Oleic acid is an endogenous ligand of TLX/NR2E1 that triggers hippocampal neurogenesis. PNAS. 119 (13), e2023784119 (2022).
- Tang, C., et al. Analytical platforms and techniques to study stem cell metabolism. Methods Mol Biol. 1842, 265-281 (2018).
- Chen, K., Baluya, D., Tosun, M., Li, F., Maletic-Savatic, M. Imaging mass spectrometry: A new tool to assess molecular underpinnings of neurodegeneration. Metabolites. 9 (7), 135 (2019).
- Zhang, H., et al. Mass spectrometry imaging for spatially resolved multi-omics molecular mapping. NPJ Imaging. 2 (1), (2024).
- Spruill, M. L., Maletic-Savatic, M., Martin, H., Li, F., Liu, X. Spatial analysis of drug absorption, distribution, metabolism, and toxicology using mass spectrometry imaging. Biochem Pharmacol. 201, 115080 (2022).
- Sekera, E. R., Akkaya-Colak, K. B., Lopez, A., Mihaylova, M. M., Hummon, A. B. Mass spectrometry imaging and histology for the analysis of budding intestinal organoids. Anal Chem. 96 (10), 4251-4258 (2024).
- Wang, Y., Hummon, A. B. MS imaging of multicellular tumor spheroids and organoids as an emerging tool for personalized medicine and drug discovery. J Biol Chem. 297 (4), 101139 (2021).
- Bakker, B., et al. Preparing ductal epithelial organoids for high-spatial-resolution molecular profiling using mass spectrometry imaging. Nat Protoc. 17 (4), 962-979 (2022).
- Cappuccio, G., Khalil, S. M., Osenberg, S., Li, F., Maletic-Savatic, M. Mass spectrometry imaging as an emerging tool for studying metabolism in human brain organoids. Front Molecular Biosci. 10, 1181965 (2023).
- Khalil, S. M., et al. MALDI Imaging mass spectrometry visualizes the distribution of antidepressant duloxetine and its major metabolites in mouse brain, liver, kidney, and spleen tissues. Drug Metab Dispos. 52 (7), 673 (2024).
- Swales, J. G., Hamm, G., Clench, M. R., Goodwin, R. J. A. Mass spectrometry imaging and its application in pharmaceutical research and development: A concise review. Int J Mass Spectrom. 437, 99-112 (2019).
- Müller, W. H., Verdin, A., De Pauw, E., Malherbe, C., Eppe, G. Surface-assisted laser desorption/ionization mass spectrometry imaging: A review. Mass Spectrom Rev. 41 (3), 373-420 (2022).
- Langridge, J. I., Claude, E. Matrix-assisted laser desorption and desorption electrospray ionization mass spectrometry coupled to ion mobility. Methods Mol Biol. 2084, 245-265 (2020).
- Khalil, S. M., Römpp, A., Pretzel, J., Becker, K., Spengler, B. Phospholipid topography of whole-body sections of the Anopheles stephensi mosquito, characterized by high-resolution atmospheric-pressure scanning microprobe matrix-assisted laser desorption/ionization mass spectrometry imaging. Anal Chem. 87 (22), 11309-11316 (2015).
- Khalil, S. M., Sprenger, R. R., Hermansson, M., Ejsing, C. S. DDA-imaging with structural identification of lipid molecules on an Orbitrap Velos Pro mass spectrometer. J Mass Spectrom. 57 (9), e4882 (2022).
- Bender, K. J., et al. Sample preparation method for MALDI mass spectrometry imaging of fresh-frozen spines. Anal Chem. 95 (47), 17337-17346 (2023).
- Paşca, A. M., et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 12 (7), 671-678 (2015).
- Qian, X., Song, H., Ming, G. Brain organoids: Advances, applications and challenges. Development. 146 (8), dev166074 (2019).
- Ferguson, C. N., Fowler, J. W. M., Waxer, J. F., Gatti, R. A., Loo, J. A. Mass spectrometry-based tissue imaging of small molecules. Adv Mass Spectrom Biomed Res. 806, 283-299 (2014).
- Wang, J., et al. MALDI-TOF MS imaging of metabolites with a N -(1-Naphthyl) Ethylenediamine Dihydrochloride matrix and its application to colorectal cancer liver metastasis. Anal Chem. 87 (1), 422-430 (2015).

.
