Measurement of Glutamate Uptake using Radiolabeled L-[3H]-Glutamate in Acute Transverse Slices Obtained from Rodent Resected Hippocampus
Summary
This article describes a reliable and simple way to obtain ex vivo acute hippocampal transverse slices from mice and rats using a tissue chopper. Slices obtained from resected hippocampi can be submitted for functional glutamate uptake analysis to investigate glutamatergic system homeostasis.
Abstract
Glutamate removal from the extracellular space by high-affinity Na+-dependent transporters is essential to ensure that the brain's intrinsic connectivity mechanisms work properly and homeostasis is maintained. The hippocampus is a unique brain structure that manages higher cognitive functions, and is the subject of several studies regarding neurologic diseases. The investigation of physiological and pathological mechanisms in rodent models can benefit from acute hippocampal slice (AHS) preparations. AHS has the advantage of providing reliable information on cell function since the cytoarchitecture and synaptic circuits are preserved. Although AHS preparations are commonly used in neurochemistry laboratories, it is possible to find some methodological differences in the literature. Considering that distinctive slice preparation protocols might change the hippocampal regions analyzed, this current protocol proposes a standard technique for obtaining transverse AHS from resected hippocampus. This simple-to-perform protocol may be used in mice and rats' experimental models and allow several ex vivo approaches investigating neurochemical dynamics (in dorsal, intermediate and ventral hippocampus) in different backgrounds (e.g., transgenic manipulations) or after in vivo manipulations (e.g., pharmacological treatments or suitable rodent models to study clinical disorders). After dissecting the hippocampus from the rodent brain, transverse slices along the septo-temporal axis (300 µm thick) were obtained. These AHS contain distinct parts of the hippocampus and were subjected to an individual neurochemical investigation (as an example: neurotransmitter transporters using their respective substrates). As the hippocampus presents a high density of excitatory synapses, and glutamate is the most important neurotransmitter in the brain, the glutamatergic system is an interesting target for in vivo observed phenomena. Thus, the current protocol provides detailed steps to explore glutamate uptake in ex vivo AHS using L-[3H]-Glutamate. Using this protocol to investigate hippocampal function may help to better understand the influence of glutamate metabolism on mechanisms of neuroprotection or neurotoxicity.
Introduction
The hippocampus, a brain structure buried deep in the medial temporal lobe of each hemisphere, where high cognitive functions lie, is one of the most studied entities of the central nervous system (CNS). The function of the hippocampus is strongly related to declarative and spatial memory. This structure also plays a part in emotional behavior and in the regulation of hypothalamic functions1,2,3,4. Ever since it was confirmed, important mechanisms of memory formation and storage take place in this region and the field began to deeply investigate the hippocampal region. Accordingly, the use of animal models that resemble human cerebral disorders related to hippocampal functions, such as Alzheimer's disease, epilepsy, major depression, and stress, continues to grow.
In rodents, the hippocampus is a curved-shaped structure starting from near the medial septum towards the ventral temporal cortex. Along its longitudinal axis, the hippocampus can be divided into three different regions, each one related to specific circuitry1. The upper part constitutes the dorsal/septal hippocampus, the lower part constitutes the ventral/temporal hippocampus, and the area between them is considered the intermediate hippocampus. There is extensive literature covering the differences in cellular projections to each part, as well as reports of specific cognitive aspects processed by each5,6. Regarding its internal organization, the hippocampal regions can be separated by its functional areas. The Cornu ammonis (CA) area is subdivided in CA1, CA2, and CA3 and extends through the superior part of the hippocampus, above the dentate gyrus (DG) and the subiculum, which are the most internal hippocampal parts (Figure 1). The synapses located in these regions undergo continuous rearrangement, showing neurogenic and plastic processes throughout life3. Several studies have already shown that distinct experimental manipulations in the hippocampus result in cognitive disability7. Regarding the assessment of biochemical and molecular alterations, techniques using acute brain slices are an excellent tool to improve the knowledge regarding different aspects of the hippocampus.
Due to its precision and reproducibility, many studies that explored aspects of neurotransmission-related phenomena (enzyme activity, uptake, or release) used transverse AHS from resected hippocampus obtained by tissue chopper8,9,10,11,12. This slicing technique followed by uptake assessment is suitable for sophisticated neurochemical experiments that require the transporter activity from hippocampal tissue to be preserved. For that, the employment of a tissue chopper is preferable, since it is faster than the vibratome and provides the AHS in a proper time for experimental use with suitable accuracy.
The excitatory neurotransmission in the brain is accomplished by glutamate, the most abundant neurotransmitter, including in the hippocampus, which is dependent on glutamate signaling to a greater extent13,14. This neurotransmitter abundance is tightly controlled in the extracellular environment. Inside intracellular vesicles, however, it can reach up to 100 mM15. Once released in the synaptic cleft, glutamate is not metabolized and needs to be removed in order to avoid excitotoxicity, usually triggered as a response to an overload of glutamate14,16. The only mechanism separating toxicity from normal signaling is sodium-dependent transport through the activity of proteins located in the plasma membranes of, majorly, glial cells14,17,18,19. These transporters [GLAST (EAAT1) and GLT-1(EAAT2)] tightly regulate extracellular glutamate levels and can be modulated by a wide range of factors, such as DNA transcription, mRNA splicing and degradation, protein synthesis and targeting, amino acid transport activity, and ion channel activities20,21,22,23. Accordingly, their activity can be measured by the transport of radiolabeled substrate, as glutamate.
The use of radiolabeled substrates represents a preferable method for quantifying transporter activity since they allow tracing dynamic mechanisms such as transport across cell membranes. Besides their high sensitivity and specificity, the advantages of radiotracer experiments include their simplicity and small expense compared to competing technologies such as mass spectrometry24. Also, by using only small amounts of tracer, physiological levels of substrates are not altered, thus providing a more representative picture of the real metabolic activity scenario.
The availability of ex vivo experimental approaches is critical to support basic research on identifies novel molecular targets and drug discovery activities. Thus, considering the relevance of the glutamate uptake for glutamatergic system homeostasis and the high predominance of glutamatergic synapses in the hippocampus, this protocol demonstrates how to assess glutamate uptake activity in a fast and easy-to-reproduce method using transverse AHS from the resected hippocampus. This assay uses radiolabeled L-[3H]-Glutamate, which allows for quantitative comparisons and clear visualization of results, and can be modified for use with specific or customized substrates, over a wide range of reaction conditions25.
Acute brain slices present many advantages and have been used to support function change under pharmacological and genetic manipulations26,27,28. Their use benefits from the following: (i) the neurochemical functionality conservation and cell-to-cell interactions; (ii) the possibility to perform numerous pharmacological and genetic manipulations to investigate pathways underlying neuronal and glial functions; (iii) precise control of the extracellular environment; and (iv) good experimental access to different hippocampal areas (such as CA1, CA3 or DG), which are kept in the same slice depending on the slicing method. Considering that distinctive slice preparation protocols might change the hippocampal regions exposed, this protocol proposes a standard technique for obtaining transverse AHS from the resected hippocampus. This simple-to-perform protocol may be used in rodent models and may allow several ex vivo approaches investigating neurochemical dynamics in different backgrounds or after in vivo manipulations29,30 (Figure 2).
Protocol
All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the local Ethics Committee (project approval # 33732/CEUA-UFRGS). All efforts were made to minimize discomfort and the number of animals used in the experiments.
1. Preparing Hank's Balanced Salt Solution (HBSS)
- In a 1 L beaker, add approximately 0.5 L of sterile or double-distilled water and begin stirring vigorously on a magnetic stirrer. Add the following HBSS components (in mM): 137 NaCl, 0.63 Na2HPO4, 4.17 NaHCO3, 5.36 KCl, 0.44 KH2PO4, 0.41 MgSO4, 0.49 MgCl2, 5.55 D-Glucose (see Table 1).
- Adjust the pH to 7.4 with NaOH or HCl before adding 1.26 mM CaCl2 to avoid precipitation. Add this salt very slowly and under agitation. Bring it up to a volume of 1 L with sterile or double-distilled water. This buffer will be used to wash the tissue and to maintain the AHS in a viable state during the experiment. It will provide inorganic ions while maintaining a physiological pH and osmotic pressure.
NOTE: The addition of divalent cations is done after titration of pH to avoid precipitation. - Separate two different fractions of HBSS: one will be kept in a water bath at 37 °C and used during the uptake paradigm, while the other will be kept ice-cold (4 °C) and used for washings before and after the experiment.
NOTE: It is possible to prepare stock solutions of each salt and keep them frozen until use, store it in the freezer (-20 °C) and use it within a few weeks.
2. Preparing Sodium-Free HBSS
- Before mixing the components for sodium-free HBSS, prepare in advance two other solutions: Glucamine-HCl (137 mM) and Glucamine-HEPES (4.17 mM). Mix equimolar concentrations of NMDG base and HCl to obtain the Glucamine-HCl solution. Do the same procedure with 2.0 M HEPES free acid to obtain the NMDG-HEPES solution (see Table 2).
- In a 0.5 L beaker, add approximately 0.2 L of sterile or double-distilled water and begin stirring vigorously in a magnetic stirrer. Add the following buffer components (in mM): 137 Glucamine-HCl, 4.17 Glucamine-HEPES, 5.36 KCl, 0.44 KH2PO4, 1.26 MgSO4, 0.41 MgCl2, and 5.55 D-Glucose (see Table 2).
- Adjust the pH to 7.4 with KOH or HCl and keep stirring. Add 1.26 mM CaCl2 very slowly and under agitation. Bring the solution up to a volume of 0.3 L with sterile or double-distilled water.
NOTE: This buffer will be used to measure non-sodium-dependent glutamate uptake, which will allow the measurement of passive/non-specific glutamate uptake. After the experiment, this parameter is subtracted from the total glutamate uptake to determine sodium-dependent uptake. - After preparation, this buffer can be stocked in the freezer (-20 °C) and used afterward in subsequent experiments. The same goes for the NMDG solutions.
- During the experiment, always keep the sodium-free HBSS ice cold.
3. Organizing the material for hippocampus dissection from rats
- Organize the dissection area next to the sink before starting the experiment. Position a specific guillotine for rats (for mice, it will be possible to use sharp scissors) into the sink to keep the bench clean.
- Arrange the surgical instruments and other materials for brain removal and hippocampal dissection in the order of use and as close as possible to where they are needed (see Table of Materials). Place the Petri dish in an ice bucket. Also, keep a plastic bag nearby for disposal of the carcass.
- Prepare both HBSS solutions at the recommended concentration for use. The sodium-free HBSS can be frozen and thawed before the experiment.
- Separate sodium-containing HBSS into two distinct portions, as previously recommended: one heated at 37 °C and another at 4 °C that will be used for dissection. Thaw sodium-free HBSS and keep at 4 °C.
- To induce anesthesia, use 3% isoflurane31. Before beginning the experiment, make sure to verify the absence of corneal reflex and nociceptive stimulation of the hind paw.
NOTE: Be careful to choose the adequate anesthetic drug, as it should not interfere with any parameters that will be under investigation. For this protocol, it is recommended the use of inhalant anesthetic (e.g., isoflurane)32.
4. Euthanizing the rat
- Euthanize the rodent by using the approved methods by the Institutional Animal Care and Use Committee (IACUC), as well as by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
- Using a guillotine, decapitate the rat immediately prior to the first cervical vertebra.
- Place the head on a clean paper towel moistened with cold saline solution. Completely remove the scalp with the surgical scissor size S, as well as the cutaneous muscle aiming to fully exposing the sutures on the superior surface of the skull. Initiate this procedure carefully, starting near the occipital bone and running posteriorly to the nasal bone.
NOTE: In case the experimental model is mice, use sharp scissors to remove the head. Be sure to cut just behind the skull to exclude excess tissue.
5. Removal of the rat brain
- Perform brain removal as quickly as possible. With an appropriate bone plier, make a single cut between both eyeholes joining both orifices in the rat's skull. After, place one tip of the surgical scissor size S into the foramen magnum and make a single cut laterally into the skull. Repeat for the other side.
- Cut the skull plates from the occipital plate, then along the midline suture of the parietal plates, and finally to the frontal plate towards the nose. Be sure that the rodent's head is held firmly and that the pressure is directed away from the brain to prevent damaging the tissue underneath.
NOTE: It is critically important that pressure is directed away from the brain to prevent damaging the tissue underneath. In young rats and mice, it is possible to cut the skull with small surgical scissors. - Gently remove the occipital and parietal plates from both hemispheres using a spatula or forceps. Carefully inspect for any dura matter that may be attached to the skull bones and stretched across the surface of the brain.
- Remove the brain immediately with the thin double-ended spatula and gently place the whole brain on an ice-cold Petri dish covered with filter paper. With the plastic Pasteur pipette dampen it with ice-cold saline solution. Immediately start to dissect the hippocampi from both brain hemispheres as explained below.
- Carefully resect the cerebellum from the whole brain with the #11 scalpel blade. In order to completely separate the cerebral hemispheres run a scalpel blade throughout the intrahemispheric fissure. With the plastic Pasteur pipette, dampen it with ice-cold HBSS buffer frequently to keep the tissue wet and preserved.
- Take each hemisphere separately, resect brain stem and midbrain from the overlying cortex with an Iris scissors. Carefully, with the surgical scissor cut the brainstem/midbrain/thalamus, while with a thin brush push down and pull these brain structures. Below it will be possible to observe the hippocampus (medial surface) and the lateral ventricle. The cortex with the hippocampus should now be free from the brainstem.
6. Hippocampi dissection from rats
- To resect the hippocampus, put one brain hemisphere in the coronal plane with the parietal cortex facing down. At this moment, it is possible to observe the white fimbria fibers that form a shallow hyperbola at the bottom of the hippocampus.
- Very carefully, with the non-dominant hand, anchor the brain tissue with thin tweezers. With your dominant hand, place other tweezers under the caudal tip of the hippocampus.
- Anchoring the medial white matter tracts, position the tweezers completely under the hippocampus to separate it from the rest of the brain gently. Apply pressure while moving the second tip slightly anteriorly and laterally. If done properly, the hippocampus should land on the filter paper.
- To finish the hippocampus dissection, remove the spare tissue with the same tweezers (usually any remaining cortex, blood vessels, and white matter). Repeat the same methodology for the second hemisphere.
- Once both hippocampi are removed, transfer it to a Petri dish with HBSS placed on the ice bucket and transfer to the cutting table area above the blade in the tissue chopper. Be sure to properly identify the dorsal and ventral areas if the investigation requires analyzing them separately.
NOTE: It is important to always distinguish the ventral and dorsal parts of the hippocampus during dissection. By doing so, the anterior end will be facing upwards33. This part of the hippocampus is slightly thinner than the posterior end.
7. Slices preparation
NOTE: While arranging the surgical material on the bench (session 2), prepare the tissue chopper to obtain the transverse slice from the resected hippocampus.
- Set the parameters in the tissue chopper before its use. The Chopper can cut slices from 100 to 400 µm; set it to 300 µm. Additionally, it is recommended a range of 60-80 strokes per minute, as it maintains the tissue integrity during the slicing procedure.
- After all the procedures performed in session 2, once the hippocampi were resected from the brain and placed on the filter paper humidified with cold saline solution on top of a smooth polypropylene support, align each hippocampus on the filter paper before submitting them to the chopping procedure.
- Place the hippocampus at a time on the circular stainless-steel cutting table from tissue chopper, perpendicular to the cutting blades to obtain the transverse hippocampus slices along the septo-temporal axis. Before starting the chopping process, lightly humidify the blade using isopropyl alcohol. This procedure keeps the tissue from sticking on the blade, allowing a more smooth and precise slicing.
- After turning the equipment on, the blade in the chopping arm will be raised and dropped, slicing the tissue in the selected thickness. This is a quick process, and normally the tissue will be properly sliced after a minute. It is possible to chop more than one hippocampus at the same time if they are kept properly separated on the cutting table.
- To separate the slices, take each sliced sample from the tissue chopper carefully using the double-ended spatula and plunge it into a Petri dish with ice-cold saline solution. Using two thin brushes, gently pinch the hippocampus and separate the slices. Be careful to maintain slice integrity through this process.
- Perform this experiment in triplicates, hence select 6 slices from each sample: 3 slices for the total glutamate uptake and 3 for the unspecific uptake.
- With the brush, gently transfer each slice from the Petri dish to a 24-well plate containing: (i) 1 mL of HBSS; and (ii) 1 mL of HBSS sodium-free. Identify the well/plate with the correspondent experimental groups and perform the analysis at least in triplicates.
NOTE: Due to a time-limited issue, if the experiment is performed in triplicates, it limits the number of animals/groups to only 6 at a time (one hippocampus per animal in 5 min uptake). This means that the L-[3H]-Glutamate will be administered every 15 s until all slices are incubated. To stop the reaction, the same interval should be observed to ensure that all samples are exposed to a 5 min incubation period.
8. Glutamate uptake assay
- During the slices stabilization in the respective buffer (HBSS or sodium-free HBSS), prepare the radioactive-containing solution by diluting L-[3H]-Glutamate in sterile water to a final concentration of 4.5 μCi/mL in a final volume that allows 20 μL per slice plus some excess, which will prevent the lack of radioactive input at the end of the batch, since it will be needed to quantify how many disintegrations per minute (DPM) was offered to each slice in the beginning.
NOTE: For each 24-well plate, it is recommended to prepare 500 μL of 4.5 μCi/mL L-[3H]- Glutamate due to the total incubation time needed for the L-[3H]-Glutamate uptake (5 min) and the time to pipette each 20 µL aliquot (0.33 μCi in each well), as explained below. The stock concentration of L-[3H]-Glutamate is usually 1.0 mCi/mL, thus prepare a 1:200 dilution to apply on the slices. - After 15 min of pre-incubation in HBSS, completely remove the buffer with a Pasteur pipette and replace it by adding to the slices 280 μL of one of the following: (i) regular HBSS (37 °C) for total uptake; or (ii) sodium-free HBSS (4 °C) for unspecific uptake. It is important to notice that total uptake must be performed on a heating plate at 37 °C, while the unspecific uptake is performed on ice.
- Prepare one timer and set the beginning of the experiment when adding 20 μL input in the first well containing a slice. Wait for 15 – 20 s (depending on the number of slices in the plate) and add the radioactive input to the next well and so on until the end of the slice-containing wells. Distribute 20 μL of input in all the wells always considering the total incubation time (5 min).
- Once each of the wells containing slices in the plate are filled, wait for the first slice to complete 5 min incubation with L-[3H]-Glutamate. Then, immediately remove the incubation media to a proper flask for radioactive waste.
- Immediately after the removal, quickly add 300 μL of ice-cold HBSS and gently remove it. Repeat this procedure two more times in each well, completing three washes for each slice. Be sure to use the sodium-free HBSS for washing the slices devoted to the unspecific uptake measurement. After washing all the slices in the plate, add 300 μL 0.5 M NaOH to each well and store the plate protected with parafilm at 4 °C overnight.
9. Counting the radioactive L-[3H]-Glutamate
- On the next day, label as many tubes as slices in the experiment and collect the content of the well to each tube. Be sure that the slices are completely solubilized in the lysis solution. If necessary, homogenize the sample using a 26-gauge needle coupled to a 1 mL syringe.
- Add 1.2 mL of scintillation liquid to a final volume of approximately 1.5 mL and homogenize all samples in a vortex before reading them in a scintillation counter for 60 s each.
NOTE: Be sure that the detection parameters are set to tritium and the result will be delivered in DPM.
10. Calculations
- Convert the results given by the scintillation counter to the radioactivity emitted by the reagent trapped within the slice and represents the amount of glutamate taken up. Deduct the value obtained for the unspecific uptake from the result obtained with the regular HBSS. One μCi is equivalent to 2.22×106 DPM.
- By knowing the amount of μCi and the original concentration of [3H]L-Glutamate (information usually provided in the reagent's flask or datasheet), calculate the L-[3H]-Glutamate captured by each slice.
Representative Results
Glutamate uptake is one of the most important mechanisms controlling neurotransmission in the brain. The hippocampus, specifically, is a critical place in glutamate signaling, being an important hub connecting memory, cognition, and emotions in the brain. Following the protocol, adult male Wistar rats were used to generate representative results. Animals were anesthetized using isoflurane 3% until unconscious. After dissecting the brain, hippocampi were removed and placed in the chopper table perpendicularly to the blade. Three hundred µm thick slices were obtained at a rate of 60 per minute and collected to a petri dish containing ice-cold saline solution. Slices were separated each in a well of a 24-well plate and L-[3H]-Glutamate uptake was carried out for 5 min (unless stated otherwise) in sodium-free or regular HBSS, at 4 or 37 °C, respectively.
After an overnight period in lysis solution at 4 °C, samples were mixed with scintillation liquid and read in a scintillation counter for 60 s. The results are given in DPM and the value of the uptake carried out in sodium-free HBSS is deducted from the value obtained with regular HBSS. One DPM corresponds to 0.000000000450 or 4,5 x 10-10 Curie (Ci). By knowing the specific radioactivity of the L-[3H]-Glutamate, is possible to establish a relationship between the result in DPM and the amount of L-[3H]-Glutamate captured by each sample.
First, we carry out the uptake assay using 200 and 300 μm slices to demonstrate that the total protein amount does not influence the uptake parameter. No differences in glutamate uptake in 5 min were observed comparing slices with 200 and 300 μm (data not shown). Accordingly, the researcher has to use slices obtained from the same hippocampal region of all animals to prevent size bias. In summary, this protocol highlights the usual thickness (300 µm) used in different experiments with slices from hippocampus, striatum or cortex10,34,35. Moreover, in order to clarify if there was any difference in L-[3H]-Glutamate uptake in the three main hippocampal regions, dorsal, intermediate and ventral regions were assayed separately. There was no difference in L-[3H]-Glutamate between the three regions (Figure 3A, p = 0.638, one-way ANOVA of repeated measures). This is not surprising since the experiments were performed with naïve animals. However, the results may vary between regions when considering different experimental models or pharmacological challenges.
To make sure that the experiment was not carried out in a saturation range, the uptake was performed for 2.5, 5, 10 or 15 min. The results demonstrate that in a 10 min incubation time the amount of L-[3H]-Glutamate taken up was significantly higher when compared with 5 min (Figure 3B, p = 0.0061, F = 6.864, one-way ANOVA of repeated measures). Conversely, 10 and 15 min uptake results were not statistically different, suggesting a plateau was reached. This data shows that a 5 min incubation is adequate to measure the uptake in most conditions.
To demonstrate that the method is sensitive enough to respond to a blockage in glutamate uptake, 100 µM dihydrokainic acid (DHK), a specific GLT-1 blocker, was administered to the AHS, 5 min prior the uptake. DHK was supplied in stabilization media during 5 min previous to media replacement and [3H]L-Glutamate uptake start36, according to the following timeline: t= 0: slice stabilization in 37 °C HBSS, t= 10 min: addition of DHK to specific slices, t= 15 min: substitution of stabilization media by fresh 37 °C HBSS and beginning of [3H]L-Glutamate uptake. As expected, DHK was able to decrease glutamate uptake (Figure 3C, p = 0.0034, r squared = 0.9069, paired Student's t test) in the protocol conditions, indicating that other pharmacological or genetic manipulations can be used to modulate glutamate uptake.
As a way to determine if the samples are stable enough to withstand a delay in the measurement, some replicates were kept with no scintillation liquid at 4 °C (Figure 3D) or frozen for 7 days (Figure 3E), having the scintillation of their counterparts measured in the next day of the experiment. The data shows that there was no difference between the DPM values of the samples tested 24 h after the experiment and the samples kept refrigerated or frozen for 7 days (p = 0.229 and p = 0.9623, respectively, paired Student's t test).
D-[3H]-Aspartate is also a substrate to glutamate transporters and represents an index of glutamate uptake, since it is metabolized in a lesser extent than L-[3H]-Glutamate. The accumulation of D-[3H]-Aspartate in the slice would provide another important index of transporters activity. Therefore, the uptake of D-[3H]-Aspartate in 300 μm hippocampal slices was estimated for 2.5 and 5 min in the same conditions than L-[3H]-Glutamate. Different time points for the uptake of L-[3H]-Glutamate and D-[3H]-Aspartate under basal conditions did not present significant differences comparing both groups (Figure 3D; p = 0.0632 for radiolabeled substrate, 2-way ANOVA followed by Tukey).
Finally, to demonstrate that unlabeled glutamate in incubation media could hamper the radiolabeled transport activity the uptake buffer was supplemented with different concentrations of unlabeled glutamate (0, 50, 100 and 200 µM). Here, in Figure 3G it is demonstrated that the presence of unlabeled glutamate in incubation media significantly impact the L-[3H]-Glutamate uptake in a concentration-dependent manner in 300 μm hippocampal slices (p = 0.02, repeated measures ANOVA).
| Reagent | Final concentration (mM) |
| CaCl2 | 1.26 |
| D-Glucose | 5.55 |
| KCl | 5.36 |
| KH2PO4 | 0.44 |
| MgCl2 | 0.49 |
| MgSO4 | 0.41 |
| NaCl | 137 |
| NaHCO3 | 4.17 |
| Na2HPO4 | 0.63 |
Table 1. Hank's Balanced Salt Solution composition
| Reagent | Final concentration (mM) |
| CaCl2 | 1.26 |
| D-Glucose | 5.55 |
| Glucamine-HCl | 137 |
| pH 7.4 | |
| (adjust with concentrated HCl) | |
| Glucamine-HEPES | 4.17 |
| pH 7.4 | |
| (adjust pH with HEPES free acid 2 M) | |
| KCl | 5.36 |
| KH2PO4 | 0.44 |
| MgCl2 | 0.49 |
| MgSO4 | 0.41 |
Table 2. Sodium-Free HBSS composition
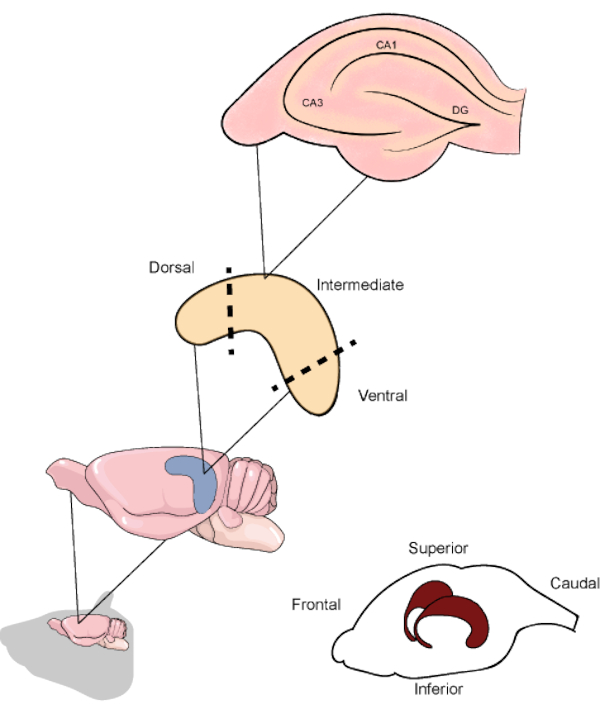
Figure 1. Schematic representation of the rodent brain and hippocampi location beneath the temporoparietal and occipital cortices. When resected, the hippocampus can be divided into the dorsal, intermediate, and ventral parts, the first being oriented in the upper direction of the brain. The hippocampal slices along the transverse axis contain the main regions (CA1, CA3 and DG). CA1: Cornu ammonis 1, CA3: Cornu ammonis 3 and DG: dentate gyrus. Please click here to view a larger version of this figure.
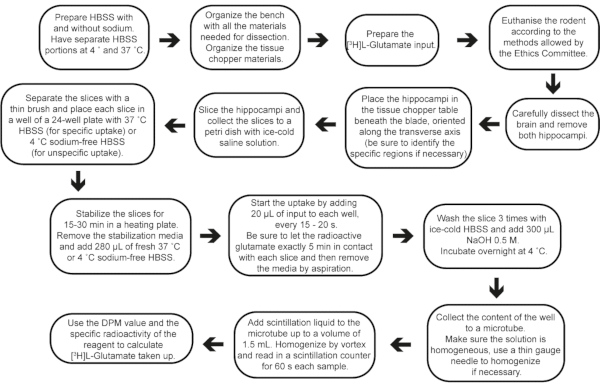
Figure 2: Step-by-step flow of the experiment, from buffer preparation to result calculations. Please click here to view a larger version of this figure.
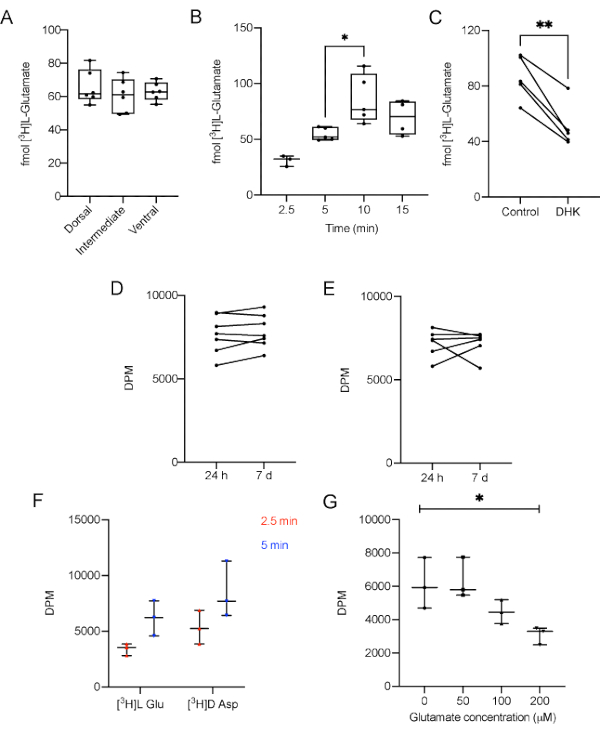
Figure 3: Representative results of L-[3H]-Glutamate uptake performed in AHS of adult male Wistar rats. A) Different regions take up the same amount of L-[3H]-Glutamate under basal conditions (p = 0.638, one-way ANOVA of repeated measures). B) The contact of L-[3H]-Glutamate with the slices for 10 min increases the amount of L-[3H]-Glutamate captured, compared with 5 min (p = 0.02, F = 6.864, one-way ANOVA). C) Dihydrokainic acid (DHK), a specific GLT-1 blocker, decreases glutamate uptake (p = 0.0034, r squared = 0.9069, paired Student's t test). Sample stability was measured after 7 days. Replicates were kept D) at 4 °C (p = 0.229, paired Student's t test) and E) -20 °C (p = 0.9623, paired Student's t test) and compared to its counterparts measured 24 h after the experiment. F) Different time points for the uptake of L-[3H]-Glutamate and D-[3H]-Aspartate under basal conditions (p = 0.0632 for radiolabeled substrate, 2-way ANOVA followed by Tukey); G) Different concentrations of unlabeled glutamate (0, 50, 100 and 200 µM) supplemented in uptake buffer (p = 0/02, one-way ANOVA of repeated measures). GLT-1: Glutamate transporter type 1. DPM: disintegrations per minute. The data in A and B are expressed as minimum value, median and maximum value. The data in C, D, and E represent paired slices, therefore they are unique values.Please click here to view a larger version of this figure.
Discussion
The presented protocol shows an easy-to-perform glutamate uptake assessment using hippocampal slices. The results demonstrate that AHS regularly takes up around 60 fmol of radiolabeled L-[3H]-Glutamate, that the thickness of the slice (protein amount) did not influence the L-[3H]-Glutamate uptake (data not shown), and that the dorsal, intermediate, and ventral parts of the hippocampus exhibited similar performances when obtained from naïve adult male Wistar rats (Figure 3A). It was also demonstrated, in a time-course experiment, that in 5 min incubation the L-[3H]-Glutamate transport system was not saturated (Figure 3B), while adding unlabeled glutamate to uptake media decreases the AHS L-[3H]-Glutamate uptake by an important reduction in DPM index (Figure 3G). Moreover, it was shown that D-[3H]-Aspartate, a radiolabeled excitatory neurotransmitter, could be also taken up in a similar way comparing to L-[3H]-glutamate (Figure 3F). Finally, an example of pharmacological manipulation was demonstrated by the incubation of DHK with AHS and as expected, a significant decrease in the final L-[3H]-Glutamate uptake was verified (Figure 3C). To test the stability of the samples, some replicates were kept at 4 °C or at -20 °C for seven days. The data indicate that the measurement of DPM did not suffer significant alterations despite the gap of time (Figure 3D, for samples kept at 4 °C; and Figure 3E, for samples kept at -20 °C).
The use of radiolabeled compounds to estimate transport across membranes is largely used in neurochemical assessments37,38. This technique has the potential to disclose a cellular mechanism underlying behavioral changes in preclinical models of several kinds of diseases. Glutamate uptake is a crucial process for brain homeostase and an essential part of neurotransmission, being able to dictate synaptic strength, length and timing. Glutamate, the most ubiquitous neurotransmitter in the brain, is involved in several complex functions, such as learning, memory, and behavior, most of which are directly related to hippocampus. Glutamatergic neurotransmission is dampened in several neurodegenerative diseases, as well as in peripheral conditions such as nutritional restrictions39. Astrocytes, glial cells responsible for the most of glutamate uptake, may undergo changes in their functions in the course of many diseases, which would impair their ability to take up glutamate at a physiological rate. The condition elicited by excess glutamate in the extracellular space, referred to as excitotoxicity, has the potential to cause extensive damage to neurons and other cells surrounding the area.
Some important observations using the AHS protocol are related to the need to normalize the L-[3H]-Glutamate uptake by the protein content. However, in acute brain slices, we hypothesize that the glutamate uptake activity occurs on the surface of the slice, and the inner tissue probably does not contribute to the amount of glutamate captured. In order to test this, we carried out uptake experiments with L-[3H]-Glutamate comparing 200 and 300 μm slices from the same animals for 5 min. It is clear to us that the use of 200 or 300 μm slices does not present significant differences in the amount of L-[3H]-Glutamate transported. Additionally, the use of 200 μm slices is experimentally more challenging since the slice is much more delicate due to its reduced thickness. Preferably, when performing this technique, the researcher should use slices obtained from the same hippocampal region of all animals.
Some studies use 5 or 7 min of uptake time10. Herein the results show a significant difference comparing 5 with 10 min (Figure 3B). In addition, a plateau was observed comparing the 10 and 15 time points. It is strongly recommended that the entire procedure is performed quickly to preserve metabolic function, reduce tissue stress and consequent damage related to the technique. Given the proper adaptations, this method can be modified to fit other neurotransmitter systems depending on the researchers' interest. AHS can be prepared from non-treated, pharmacologically treated, and/or genetically modified animals. Additionally, it allows ex vivo manipulations such as treatment of the tissue with compounds that modulate neurotransmitter uptake. Combining these approaches allows testing a variety of research applications40. As expected, unlabeled glutamate in incubation media decreases L-[3H]-Glutamate uptake in a concentration-dependent manner (Figure 3G).
Considering that glutamate transporters can take up both D- and L-Aspartate, we performed an experiment comparing the L-[3H]-Glutamate and D-[3H]-Aspartate uptake. No differences were observed (Figure 3F); however, it is important to highlight that D-[3H]-Aspartate is metabolized in a lesser extent than L-[3H]-Glutamate. Thus, the accumulation of D-[3H] -Aspartate in the slice could provide another important parameter to measure transporter activity.
The present results reported the total amount of L-[3H]-Glutamate taken up by each AHS. It is important to note, however, that one of the most common ways of reporting L-[3H]-Glutamate is dividing the amount taken up by the time, thus reporting in fmol/min. Also, when using cell culture to L-[3H]-Glutamate uptake, it is common to measure the protein of the sample and report the results as fmol L-[3H]-Glutamate /mg protein/min. When using AHS or any other brain slices, it is not recommended to report the results referring to the protein, since a 300 µm slice contains much more protein than the protein that indeed may take up the L-[3H]-Glutamate.
Regarding this protocol, it is still important to clarify some important issues. i) HBSS will be used to measure total specific glutamate uptake, as the glutamate transport is sodium-dependent. ii) N-methyl-D-glucamine (NMDG) chloride is frequently used as a substitute for NaCl in systems to maintain physiological solutions as isotonic without sodium. iii) The addition of HEPES to this formulation may improve the AHS's resistance to brain slicing edema and provide stronger pH buffering38. iv) The use of salts containing K+ should be avoided since high concentrations of K+ in the extracellular medium can interfere with membrane potential. Therefore, KCl should not be used as an alternative to NaCl; and v) to induce unconsciousness, the rats were exposed to isoflurane 3% before euthanasia for at least one minute. Considering that it has been previously shown that isoflurane influences glutamate uptake41, it is argued that the time taken between isoflurane exposure and ex vivo glutamate uptake had no significant effect on the parameters illustrated in this protocol.
One limitation of the technique is that it does not distinguish how much glutamate is taken up by glial and neuronal transporters. Nevertheless, the contribution of neuronal transporters in the glutamate uptake is rather modest, and most prominent transporters are located on astrocytes surrounding synapses14,17. Also, the assessment of glutamate uptake in specific, almost microscopic, hippocampal areas (e.g., CA3 exclusively) would pose an additional challenge, since the dissection procedure would be much more delicate. It is possible to decrease inter-experimental variation by performing the hippocampal slicing quickly (less than 3 minutes) and maintaining the tissue humidified with HBSS buffer or saline solution until placing it in the plate for the assay. Another advantage of using this protocol is the possibility to perform kinetics experiments, since it was chopped 3 whole hippocampi from adult rats and counted between 34-36 slices from each one. This way, with a single adult rat it is possible to obtain about 70 transverse hippocampal slices from both hippocampi and different areas (ventral, intermediate and dorsal). Therefore, this protocol provides a complete picture of the glutamate uptake by the slices obtained from resected hippocampus.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Authors receive financial support from the Brazilian National Institute of Science and Technology in Excitotoxicity and Neuroprotection [465671/2014-4], CNPq [438500/2018-0], and [152189/2020-3], FAPERGS/CAPES/DOCFIX [18/2551-0000504-5], CAPES [88881.141186/ 2017-01], CNPq [460172/2014-0], PRONEX, FAPERGS/CNPq [16/ 2551-0000475-7], FAPERGS/MS/CNPq/SESRS-PPSUS [30786.434.24734.23112017]
UFOP – MODALIDADE: "EDITAL PROPP 19/2020 AUXÍLIO À PUBLICAÇÃO DE ARTIGOS CIENTÍFICOS – 2020", PROCESSO N.: 23109.000929/2020-88
Materials
| #11 scalpel blade | Swann-Morton | 525 | |
| 100 mm glass petri dish | Common suppliers | ||
| 110 mm diameter Whatman Filter | Sigma Aldrich | WHA1001110 | |
| 42.5 mm diameter Whatman Filter | Sigma Aldrich | WHA1001042 | |
| 24-well cell culture plate | Falcon | 353047 | |
| Becker | Common suppliers | ||
| Blades for the tissue chopper | Wilkinson | 3241 | |
| Bone rongeur | Erwin Guth | 9,00,005 | |
| CaCl2 | Sigma Aldrich | C4901 | |
| D-[2,3-3H]-Aspartic acid | PerkinElmer | NET581001MC | 11.3 Ci/mmol (37 MBq) |
| D-Glucose | Sigma Aldrich | G8270 | |
| N-Methyl-D- Glucamine | Sigma Aldrich | M2004 | |
| HEPES | Sigma Aldrich | H3375 | |
| Hidex 300 SL | Hidex Oy. | Super Low Level #425-020 | |
| Iris scissors | Erwin Guth | 8,00,040 | |
| Isoflurane | Cristalia (São Paulo, Brazil) | 4,10,525 | 1 mL/mL |
| KCl | Sigma Aldrich | P3911 | |
| KH2PO4 | Sigma Aldrich | P0662 | |
| L-[3,4-3H]-Glutamic Acid | PerkinElmer | NET490005MC | 49.7 Ci/mmol (185 MBq) |
| MgCl2 | Sigma Aldrich | M8266 | |
| MgSO4 | Sigma Aldrich | M7506 | |
| Na2HPO4 | Sigma Aldrich | S9763 | |
| NaCl | Sigma Aldrich | S9888 | |
| NaHCO3 | Sigma Aldrich | S5761 | |
| Plastic Pasteur pipette | Common suppliers | ||
| Scintillation liquid | PerkinElmer | 1200.437 for 1 x 5 Liter | Optiphase HiSafe 3 |
| Small surgical scissors | Erwin Guth | 8,00,040 | |
| Small tweezers | Erwin Guth | 6,00,131 | |
| Spare chopping discs for the chopper | Common suppliers | ||
| Standard scissors | Erwin Guth | 8,00,010 | |
| Thin brushes (size 0 or 2) | Common suppliers | ||
| Thin double-ended spatula | Erwin Guth | 470.260E | |
| Tissue Chopper | Ted Pella, Inc. | 10180 |
References
- Knierim, J. J. The hippocampus. Current Biology. 25 (23), 1116-1121 (2015).
- Stella, F., Cerasti, E., Si, B., Jezek, K., Treves, A. Self-organization of multiple spatial and context memories in the hippocampus. Neuroscience & Biobehavioral Reviews. 36 (7), 1609-1625 (2012).
- Toyoda, H., et al. Interplay of amygdala and cingulate plasticity in emotional fear. Neural Plasticity. 2011, 813749 (2011).
- Koehl, M., Abrous, D. N. A new chapter in the field of memory: adult hippocampal neurogenesis. European Journal of Neuroscience. 33 (6), 1101-1114 (2011).
- Bannerman, D. M., et al. Regional dissociations within the hippocampus–memory and anxiety. Neuroscience & Biobehavioral Reviews. 28 (3), 273-283 (2004).
- Moser, E., Moser, M. B., Andersen, P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. Journal of Neuroscience. 13 (9), 3916-3925 (1993).
- Spiers, H. J., Bendor, D. Enhance, delete, incept: manipulating hippocampus-dependent memories. Brain Research Bulletin. 105, 2-7 (2014).
- Gubert, P., et al. Low concentrations of methamidophos do not alter AChE activity but modulate neurotransmitters uptake in hippocampus and striatum in vitro. Life Sciences. 88 (1-2), 89-95 (2011).
- Andersen, J. V., et al. Extensive astrocyte metabolism of gamma-aminobutyric acid (GABA) sustains glutamine synthesis in the mammalian cerebral cortex. Glia. 68 (12), 2601-2612 (2020).
- Nonose, Y., et al. Guanosine enhances glutamate uptake and oxidation, preventing oxidative stress in mouse hippocampal slices submitted to high glutamate levels. Brain Research. 1748, 147080 (2020).
- Rambo, L. M., et al. Creatine increases hippocampal Na(+),K(+)-ATPase activity via NMDA-calcineurin pathway. Brain Research Bulletin. 88 (6), 553-559 (2012).
- Papp, L., Vizi, E. S., Sperlagh, B. Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor-/- mice. Neuroreport. 15 (15), 2387-2391 (2004).
- Amaral, D. G., Witter, M. P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. 神经科学. 31 (3), 571-591 (1989).
- Danbolt, N. C. Glutamate uptake. Progress in Neurobiology. 65 (1), 1 (2001).
- Featherstone, D. E. Intercellular glutamate signaling in the nervous system and beyond. ACS Chem Neurosci. 1 (1), 4-12 (2010).
- Dong, X. X., Wang, Y., Qin, Z. H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmaceutica Sinica B. 30 (4), 379-387 (2009).
- Grewer, C., Rauen, T. Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. Journal of Membrane Biology. 203 (1), 1-20 (2005).
- Tzingounis, A. V., Wadiche, J. I. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nature Reviews Neuroscience. 8 (12), 935-947 (2007).
- Vandenberg, R. J., Ryan, R. M. Mechanisms of glutamate transport. Physiological Reviews. 93 (4), 1621-1657 (2013).
- Peterson, A. R., Binder, D. K. Post-translational Regulation of GLT-1 in Neurological Diseases and Its Potential as an Effective Therapeutic Target. Frontiers in Molecular Neuroscience. 12, 164 (2019).
- Martinez-Lozada, Z., Guillem, A. M., Robinson, M. B. Transcriptional Regulation of Glutamate Transporters: From Extracellular Signals to Transcription Factors. Advances in Pharmacology. 76, 103-145 (2016).
- Carbone, M., Duty, S., Rattray, M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochemistry International. 60 (1), 31-38 (2012).
- Jimenez, E., et al. Differential regulation of the glutamate transporters GLT-1 and GLAST by GSK3beta. Neurochemistry International. 79, 33-43 (2014).
- Wang, C. H., et al. . Radiotracer Methodology in the Biological, Environmental and Physical Sciences. , (1975).
- Karra, A. S., Stippec, S., Cobb, M. H. Assaying Protein Kinase Activity with Radiolabeled ATP. Journal of Visualized Experiments. (123), e55504 (2017).
- Buskila, Y., et al. Extending the viability of acute brain slices. Scientific Reports. 4, 5309 (2014).
- Cho, S., et al. Spatiotemporal evidence of apoptosis-mediated ischemic injury in organotypic hippocampal slice cultures. Neurochemistry International. 45 (1), 117-127 (2004).
- Pringle, A. K., Sundstrom, L. E., Wilde, G. J., Williams, L. R., Iannotti, F. Brain-derived neurotrophic factor, but not neurotrophin-3, prevents ischaemia-induced neuronal cell death in organotypic rat hippocampal slice cultures. Neuroscience Letters. 211 (3), 203-206 (1996).
- Paniz, L. G., et al. Neuroprotective effects of guanosine administration on behavioral, brain activity, neurochemical and redox parameters in a rat model of chronic hepatic encephalopathy. Metabolic Brain Disease. 29 (3), 645-654 (2014).
- Hansel, G., et al. The potential therapeutic effect of guanosine after cortical focal ischemia in rats. PLoS One. 9 (2), 90693 (2014).
- Cittolin-Santos, G. F., et al. Neurochemical and Brain Oscillation Abnormalities in an Experimental Model of Acute Liver Failure. 神经科学. 401, 117-129 (2019).
- Westphalen, R. I., Hemmings, H. C. Effects of isoflurane and propofol on glutamate and GABA transporters in isolated cortical nerve terminals. Anesthesiology. 98 (2), 364-372 (2003).
- Lee, A. R., Kim, J. H., Cho, E., Kim, M., Park, M. Dorsal and Ventral Hippocampus Differentiate in Functional Pathways and Differentially Associate with Neurological Disease-Related Genes during Postnatal Development. Frontiers in Molecular Neuroscience. 10, 331 (2017).
- Thomazi, A. P., et al. Ontogenetic profile of glutamate uptake in brain structures slices from rats: sensitivity to guanosine. Mechanisms of Ageing and Development. 125 (7), 475-481 (2004).
- Nonose, Y., et al. Cortical Bilateral Adaptations in Rats Submitted to Focal Cerebral Ischemia: Emphasis on Glial Metabolism. Molecular Neurobiology. 55 (3), 2025-2041 (2018).
- Arriza, J. L., et al. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. Journal of Neuroscience. 14 (9), 5559-5569 (1994).
- Aggarwal, S., Mortensen, O. V. In Vitro Assays for the Functional Characterization of the Dopamine Transporter (DAT). Current Protocols in Pharmacology. 79, 12 (2017).
- MacGregor, D. G., Chesler, M., Rice, M. E. HEPES prevents edema in rat brain slices. Neuroscience Letters. 303 (3), 141-144 (2001).
- Moreira, J. D., et al. Dietary omega-3 fatty acids prevent neonatal seizure-induced early alterations in the hippocampal glutamatergic system and memory deficits in adulthood. Nutritional Neuroscience. , 1-12 (2020).
- Cho, S., Wood, A., Bowlby, M. R. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Current Neuropharmacology. 5 (1), 19-33 (2007).
- Zuo, Z. Isoflurane enhances glutamate uptake via glutamate transporters in rat glial cells. Neuroreport. 12 (5), 1077-1080 (2001).

.
