Automatically Generated
Establishment of a Novel Ex Vivo Lung Perfusion System for Rat Lungs After Circulatory Death
Summary
The objective is to develop a new ex vivo lung perfusion (EVLP) model in rats that can sustain consistent lung function for 4 h throughout EVLP and furnish the industry with implementation specifics that will offer a sturdy method for applying EVLP to rodent models.
Abstract
The increasing scarcity of donor lungs for transplantation has spurred significant interest in the utilization of organs procured from donors after cardiac death (DCD). However, a critical challenge remains in maximizing the number of viable lungs that meet stringent criteria for clinical use. Ex vivo lung perfusion (EVLP) emerges as a promising technology to evaluate and potentially improve the function of donated lungs. This paper describes the development of a cost-effective and reproducible small animal experimental platform specifically designed for EVLP research. This platform offers a high degree of customization, enabling researchers to tailor experimental parameters to address specific research questions within the field of EVLP. The team established a rat model to simulate DCD and implemented a two-stage preservation approach. Lungs were subjected to static cold storage using a perfusate solution at 4 °C for 4 h, followed by normothermic EVLP for another 4 h. Researchers evaluated various lung function parameters, including pulmonary vascular resistance, pulmonary dynamic compliance, blood gas measurements of the perfusate, and apoptotic cell death in lung tissues. The study demonstrates that EVLP could significantly improve the functional performance of DCD lungs compared to simple cold storage at 4 °C, suggesting that EVLP has the potential to mitigate ischemia-reperfusion injury and enhance graft function following circulatory death, thereby increasing the pool of transplantable lungs.
Introduction
Lung transplantation (LTx) remains the only definitive treatment for patients with end-stage lung disease. The success of lung transplantation hinges on the availability of high-quality donor lungs with adequate function. However, a growing waitlist for lung transplants has emerged globally due to a critical shortage of donor lungs1. Many patients on the waitlist experience a decline in health or even death while waiting for a suitable donor lung within a reasonable timeframe2. Therefore, it is crucial to expand the pool of potential grafts to meet the needs of patients on the transplant waiting list. Donation after cardiocirculatory death (DCD)1,3 and ex vivo lung perfusion (EVLP)4,5 are two promising strategies to increase the number of available lungs for transplantation. Currently, donation after brain death (DBD)6 is the primary source of lung transplants. However, due to the current graft shortage, DCD is gaining traction. This shift is driven by the limitations of DBD grafts, where changes in blood flow, mechanical ventilation, infection, and aspiration can compromise graft quality. The quality of the graft directly impacts patient outcomes and long-term survival after transplantation. The ex vivo lung perfusion (EVLP) process effectively increased the number of available lung transplants by reassessing lung grafts from donors after cardiac death (DCD), resulting in similar results in both the short and long-term7,8. The goal of this study was to develop an extracorporeal lung perfusion system in rats with cyclically dead lungs and accurately assess DCD donors. To decrease the likelihood of primary graft dysfunction (PGD), the harm to DCD donors9 may be minimized by using molecular diagnostics10 and employing specialized targeted treatments.
EVLP is a technique that replicates the natural environment of the lungs outside the body, maintaining a normal physiological temperature. It allows for continuous monitoring and assessment of the lungs11,12, extends the preservation time of transplants13, and uses external perfusion, ventilation, and medications to improve the quality of marginal lungs, making them suitable for transplantation14. The first successful use of marginal donor lungs for lung transplantation using EVLP was reported by Steen et al. in 200715. Since then, the use of EVLP technology has gradually become integrated into clinical practice16,17. The Toronto team subsequently adapted the EVLP approach by using low-tidal volume ventilation and low flow rates in conjunction with a centrifugal pump's ideal lung protection strategy; this resulted in steady perfusion for a duration of 12 h18.
EVLP offers several benefits, including reducing the risk of primary graft failure, minimizing pulmonary edema, mitigating brain death-related inflammation, preparing the recipient's immune system for transplantation, and preventing lung infections, aspiration, pulmonary embolism, and ischemia-reperfusion injury14,19,20. While EVLP offers significant advantages, there is a need for improved methods to enhance graft quality.
Currently, there is no consensus on the specific settings for perfusion and ventilation in the rat EVLP platform. Ohsumi et al.21 devised a new model to study how donor lungs that were put through long periods of cold ischemia time (CIT) could keep their lung function during the ex vivo lung perfusion (EVLP) process and after being transplanted. Internationally, there have been several efforts to create rat ex vivo lung perfusion (EVLP) models22,23,24,25,26,27 in order to achieve more reliable and precise perfusion. Therefore, it is essential to develop a cost-effective, highly reproducible, and tightly controlled small animal EVLP system to facilitate further research in this area. Existing EVLP systems for small animals are often expensive and have limited flexibility in terms of modifying operating conditions for different research needs. This paper proposes a cost-effective and adaptable EVLP system design. Furthermore, it provides a detailed explanation of the technical and surgical steps required for its implementation. We employed a model utilizing deceased rats in circulatory arrest to demonstrate that EVLP improves the quality of DCD lungs, thereby increasing the pool of viable lung donors for transplantation. We aim to share our successful experience to assist other researchers in establishing a readily available EVLP platform for small animals following cardiocirculatory death.
Protocol
The experimental animals were SD male rats weighing 250-400 g. The rats were housed under standard experimental conditions at 18-22 °C with a controlled 12 h light-dark cycle and had free access to food and water. The Animal Care and Use Committee of the Institute of Clinical Research of China-Japan Friendship Hospital approved the animal experiments and conducted them in accordance with the guidelines. All surgeries were performed under general anesthesia. Figure 1 presents a schematic diagram and an actual image of the EVLP system for reference. The specific make and model of all instruments and equipment used are detailed in the Table of Materials
1. Apparatus construction
NOTE: 3D-printed plastic appears to be a promising choice for the apparatus. However, based on our limited experience, it is difficult to regulate the force while tying knots with plastic materials; too little force might cause the tube to slip off, and too much force can block the plastic tubing. Metal rigid tubes avoid these issues and are relatively durable, so these are used here.
- Connect the module to the plastic pipe and use an iron frame stand and adjustable lifting platform to position all parts at the appropriate height. Add 150 mL of perfusion into the liquid storage tank, open the peristaltic pump, and set the flow rate to 20 mL/min to make the perfusion fill the tubing.
- 1.2Grind a circular groove for endotracheal intubation. Prepare a gas mixture containing 8% O2, 6% CO2, and 86% N2 as a hypoxic gas. Pass hypoxic gas into the perfusion through the artificial membrane lung. Ventilate with pure oxygen for 5 minutes prior to blood gas measurement.
- Make the pulmonary artery and atrial cannulas out of hollow 304 stainless steel tubes that are curved to fit the anatomy of rats (Figure 2A). Grind a circular groove for artery intubation. Make a hole at the distal end of the atrial cannula wall and polish the annular groove on the external wall (Figure 2C). Set an exhaust device before the tubing into the pulmonary artery perfusion tubing.
- Connect a three-way valve to the external circulation system of the water bath and regulate the temperature of the reservoir, organ compartment, and pulmonary artery perfusate. Minimize the length of the non-water bath connection pipe and control the temperature error at all points of the device within the range of ± 0.2 °C. The desired temperature for the study design is 37 °C.
- Fill the pressure monitoring line with saline and adjust the pressure monitoring device to the same height as the donor's lung. Open the valve to connect the pressure monitoring line with the pulmonary artery and venous perfusion line, start the data collector, and set zero. Monitor arterial perfusion pressure and venous return pressure in real-time.
2. Rat anesthetization
- Wear personal protective equipment: surgical masks, gloves, and disposable overalls before handling rats and rat tissues.
- Weigh the rats and record their weights. Fill a syringe with 1000 U/kg of heparin.
- Place the rats in a closed induction chamber and anesthetize them with 3% isoflurane gas. Clamp the rats' toes; if there is no response, it indicates that the rats have reached the desired depth of anesthesia. Otherwise, continue increasing the anesthesia time. Apply vet ointment to prevent eye dryness.
- Prepare multiple sutures (3-0 or 4-0). Transfer the rats to the operating table, position them in a supine position, secure them with sutures, and disinfect them with alcohol spray.
3. Donor lung procurement
- Place the rat's head in the anesthesia mask and maintain anesthesia by delivering air containing 1.5% isoflurane.
- Make a 2-3 cm longitudinal incision with rodent surgical scissors along the midline of the neck in front of the trachea, cut the skin, dissect the muscle tissue in front of the trachea, and fully expose the trachea.
- Free the space behind the trachea, pass a 3-0 suture through it and tie a loose knot for later use. Make a V-shaped incision 0.5 cm with rodent surgical scissors above the knot in the trachea, insert a tracheal tube (14G) into the trachea, tighten the suture knot, and secure the tracheal tube (Figure 3A).
- Connect the tracheal tube to the ventilator, turn it on, and begin ventilation of the lungs.
- Stop ventilation. Monitor and record vital signs every 5 min. Confirm death after a 5 min observation period following cardiac arrest.
- Initiate lung recruitment maneuvers, restart mechanical ventilation in pressure-controlled mode, and ventilate for 5 min. Use the following parameters: PEEP 3 cmH2O, inspiratory: expiratory (I:E) ratio 1:2, respiratory rate 40 breaths/min, and FiO2 0.5.
- Clamp the trachea and allow the lungs to undergo 1 h of warm ischemic time (WIT). Following the WIT period, extract the lungs as described below and randomly divide them into three groups: (1) direct lung harvest, (2) 4 h cold static preservation (CS), and (3) 4 h EVLP evaluation.
- For direct lung harvest, sample the obtained donor lungs without any treatment for various tests. For 4 h cold static preservation (CS), store donor lungs in cold static preservation in the refrigerator at 4 °C for 4 h and then sample for detection. 4 h EVLP evaluation. For 4 h EVLP evaluation, follow step 5.
- Adjust the operating table to a 45° head-high and feet-low inclined position.
- Remove the hair with a hair shaver from the middle of the rat's chest and abdomen, disinfect it with iodine 3x, and drape it with a surgical towel.
- Make a 6-7 cm longitudinal incision along the abdomen's midline with rodent surgical scissors, cut the skin, open the abdominal wall to the abdominal cavity, and expose the abdominal organs. Use a cotton swab to gently push the intestines to the right side of the abdominal cavity to expose the inferior vena cava behind them.
- Using a syringe, inject a solution of 1000 U/kg of heparin into the inferior vena cava. Wait for 5 min to ensure adequate heparinization of the blood. Heparin prevents microthrombi formation in the pulmonary artery.
- Cut the rat's inferior vena cava with scissors, exsanguinating it. Start mechanical ventilation of the lungs with the initial ventilator settings at a tidal volume of 3 mL/kg, positive end-expiratory pressure (PEEP) of 2 cmH2O, respiratory rate of 60 breaths/min, and an I: E ratio of 1:2.
- Use forceps to lift the xiphoid process; make a longitudinal incision along the sternum from bottom to top. Use a rib spreader to expose the chest cavity. Remove the thymus tissue to expose the heart and the major blood vessels below it.
NOTE: Rat lungs are fragile; avoid touching them or abdominal organs with metallic instruments. For better visibility, gently push the lungs aside with a cotton swab. - Free the rat's aorta and the space behind the pulmonary artery, pass a 3-0 suture through it, and tie a loose knot for later use.
- Make a 2-3 mm V-shaped incision on the anterior surface of the right ventricle outflow tract, insert a pulmonary artery cannula into the pulmonary artery through the incision, and tighten the pre-tied suture to secure the cannula (Figure 3B).
- Cut the rat heart tip, insert hemostatic forceps into the left ventricle, disrupt the valves between the left ventricle and left atrium, and ensure the left atrial outflow tract is clear.
- Connect the pulmonary artery cannula to the perfusion circuit and flush the residual blood at a rate of 0.6-1 mL/min using 15 mL of low-potassium solution. Flush until the lungs turn white or until the perfusion fluid coming out of the left ventricle turns white.
- Place a suture behind the heart and encircle the ventricle; insert the left ventricle cannula through the incision in the left ventricle; and tighten the pre-tied suture to secure the cannula (Figure 3C).
- Cut off the trachea above the tracheal tube, lift the trachea, and use scissors to separate the connective tissue behind the trachea downward to the level of the diaphragm.
- Cut off the inferior vena cava and the main pulmonary artery above the diaphragm; remove the heart and lungs (Figure 3D).
- To keep the lungs inflated, immediately clamp the lower third of the trachea at the end of inhalation, collect the heart and lungs, and place them in a low potassium solution for preservation.
- Place the heart and lungs in the EVLP circuit's designated position and connect the left ventricle cannula to the perfusion circuit (Figure 4A).
4. Initialization of the ex vivo lung perfusion system
- Assemble the EVLP circuit: connect the tubing, oxygenator, reservoir, peristaltic pump, and organ chamber.
- Fill the EVLP circuit with 120 mL of lung perfusion repair solution that has 1000 units of sodium heparin, 50 mg of cefoperazone, and 50 mg of methylprednisolone before perfusion. Get rid of any air bubbles in the tubing so as to use the exhaust device at the end of the pulmonary artery perfusion.
- Set the water bath to room temperature and start circulating warm water to heat the entire EVLP circuit.
- Open the computer's data acquisition program and connect the pulmonary artery pressure sensor, lung weight sensor, pump speed sensor, and temperature sensor to the EVLP circuit and the Recorder.
- Fill the blood pressure monitoring tubing with normal saline, adjust the table's height to match the height of the supplied lung, connect the pressure monitoring tubing to the pulmonary artery and venous perfusion tubing, open the valve, start the data collector, and zero the system.
- Open the hypoxic gas valve, pass hypoxic gas through the membrane lung for 10 min until the perfusion solution reaches a stable hypoxic state, close the gas valve, and pause the peristaltic pump, awaiting the arrival of the donor lung for ex vivo lung perfusion.
- Prepare the operating table and surgical instruments near the small animal EVLP platform and pre-sterilize them with high-pressure steam sterilization.
5. Ex vivo lung perfusion
- Fill the bubble trap with a sufficient amount of perfusion solution to prevent bubbles from entering the lungs (causing air embolism).
- Place the cardiopulmonary unit in the organ chamber, connect it to the EVLP device, turn on the ventilator and peristaltic pump, and start EVLP perfusion (Figure 4B).
- Start perfusion at 20% of the target flow rate; gradually increase the flow rate to the target flow rate within 1 h; calculate the target flow rate as 20% of the cardiac output (75 mL/min/250 g donor weight).
- Set the heat exchanger to 40 °C to maintain a lung temperature of 37.5 °C. Within 20 min, the temperature of the perfusion solution increases from 25°C to 37°C.
- Remove the endotracheal clamp 20 min after the start of perfusion. Start mechanical ventilation with a respiratory rate (RR) of 60 bpm, positive end-expiratory pressure (PEEP) of 3 cmH2O, and an initial tidal volume (VT) of 4 mL/kg, reaching a maximum tidal volume of 6 mL/kg within 10 min.
- Start the flow of hypoxic gas (8% CO2, 6% O2, and 86% N2) to maintain the perfusion solution PCO2 entering the pulmonary artery between 35 and 45 mmHg simultaneously during ventilation.
- Monitor the perfusion solution flow rate, arteriovenous pressure, airway peak pressure, and lung function parameters constantly during perfusion.
Representative Results
Evaluating the number of apoptotic cells detected by TUNEL staining, the levels of inflammatory markers (detected in the perfusate using the QAH-INF-1 assay kit), and the general physical appearance (Stained with hematoxylin and eosin (H&E)) of heart-beating donor lungs in all groups is necessary to determine how well EVLP preserved DCD donor lungs. All DCD donor lungs underwent 4 h of perfusion. Most lungs exhibited stable compliance, with a gradual decrease observed over the 4 h period (Figure 5B). No significant differences were found in vascular resistance and pulmonary graft oxygenation levels among the DCD donor lungs (Figure 5A,C). Glucose levels in the perfusate declined over time, with no significant differences observed between individual rats (Figure 5D). Electrolyte levels in the perfusate remained similar across groups (Figure 5E-F).
TUNEL staining was performed using the terminal deoxynucleotidyl transferase dUTP nick end labeling technique with the apoptosis detection kit, following the manufacturer's instructions for apoptotic nuclear detection. TUNEL staining was employed to quantify apoptosis (programmed cell death) of alveolar epithelial cells in DCD donor lungs under different preservation conditions. Blinded analysis of apoptotic cell numbers revealed a significantly higher number of positive cells in the donor group compared to other groups. Additionally, the cold preservation group exhibited a higher number of positive cells compared to the EVLP-perfused DCD donor lungs (Figure 6). Five independent variables were used to score the pathological sections stained by H&E: 1) inflammatory cell influx in alveolar space, 2) alveolar septal thickening, 3) intra- and extra-alveolar hemorrhage, 4) intra-alveolar edema, and 5) hyaline membrane formation. Variables were scored from 0-4:0 = negative, 1 = slight, 2 = moderate, 3 = high and 4 = severe. The sum of the scored variables generated the total lung injury scores. The Lung injury score is significantly lower in the EVLP group than in the cold static preservation group and the control group (Figure 7A). Alveolar wall thickening and alveolar hemorrhage can be observed in the 4 h cold static preservation group (Figure 7B). The normal alveolar structure is maintained in the EVLP group (Figure 7C).
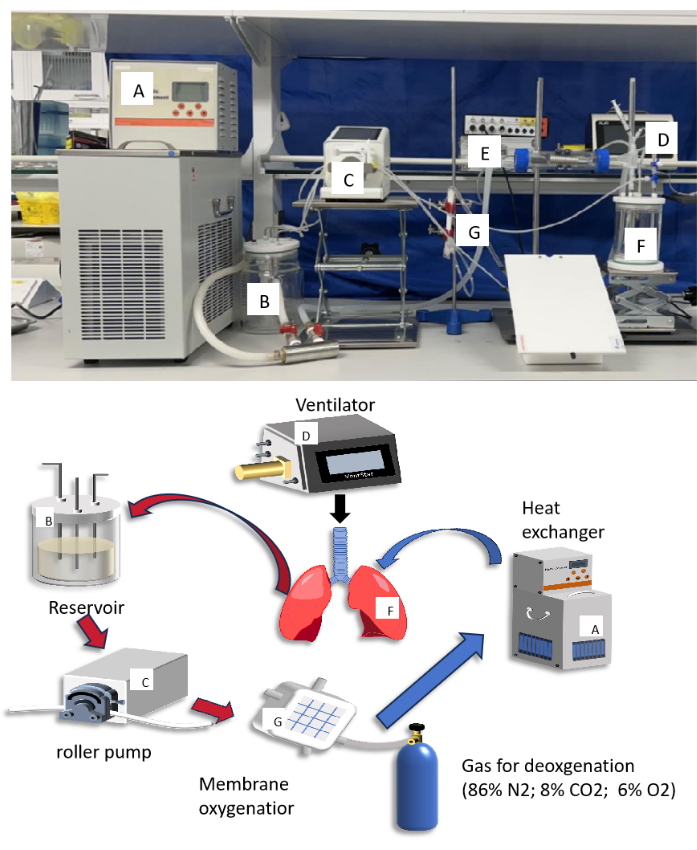
Figure 1: Diagram and photograph of small animal ex vivo lung perfusion (EVLP) circuit. The letters in the diagram matched up with the letters in the photograph to describe what each component does. Please click here to view a larger version of this figure.
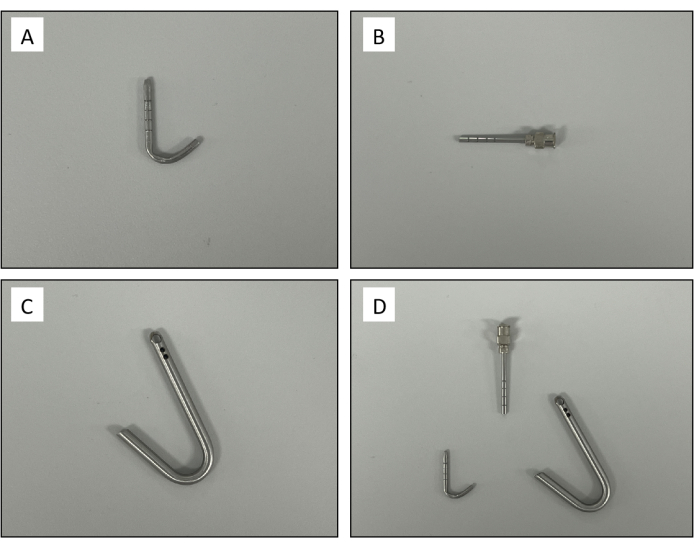
Figure 2: Custom-made cannulas. (A) Pulmonary artery cannula. (B) Trachea cannula. (C) Left atrium cannula. (D) A set of rat ex vivo lung perfusion cannula. Please click here to view a larger version of this figure.
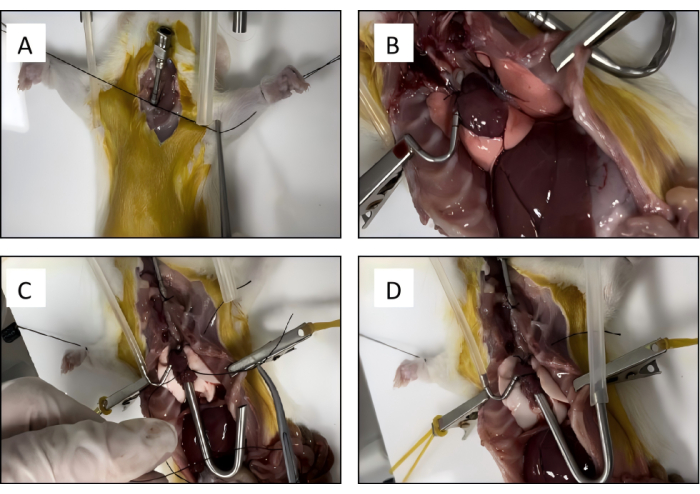
Figure 3: Rat lung isolation. (A) The trachea cannula is placed into position and secured with a silk suture. (B) The pulmonary artery is cannulated and tied with the previously placed silk suture. (C) The left atrium cannula is secured with a silk suture. (D) The connective tissue is bluntly dissected to remove the heart-lung block. Please click here to view a larger version of this figure.
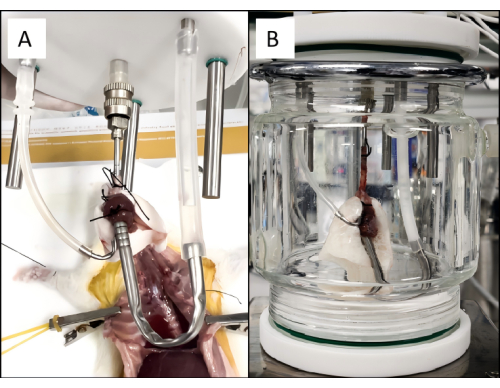
Figure 4: Rat pulmonary perfusion setup. (A) The heart-lung block is placed into the ex vivo lung perfusion circuit. (B) A properly ventilating and perfusing lung connected to the EVLP circuit. Please click here to view a larger version of this figure.
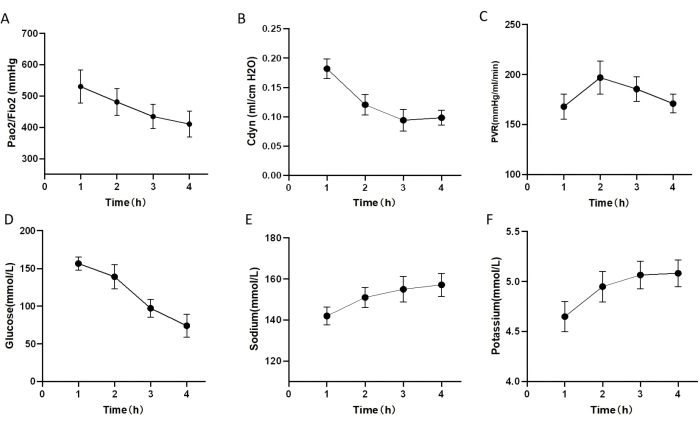
Figure 5: Lung function during rat EVLP protocol. (A-F) Lung function and blood gas analysis of donor lungs undergoing EVLP after exposure to 1 h warm ischemic time (WIT). (A) Pulmonary oxygenation index. (B) Dynamic lung compliance (Cdyn). (C) pulmonary vascular resistance (PVR). (D) The glucose level in perfusion 4 h after EVLP. (E) The sodium level in perfusion 4 h after EVLP. (F) Potassium level in perfusion 4 h after EVLP. The error bars indicate standard deviation. Please click here to view a larger version of this figure.

Figure 6: Representative images of TUNEL assay of different cases. TUNEL-positive cells: Green fluorescence shows apoptotic cells, DAPI: Blue fluorescence represents the nucleus, Merge: All cells, including cells undergoing programmed cell death (apoptosis); DCD refers to the group of direct lung harvest. DCD+COLD refers to the group of 4 h cold static preservation. DCD+EVLP refers to the group of 4 h ex vivo lung perfusion (EVLP). Please click here to view a larger version of this figure.

Figure 7: Lung injury score and histological findings. (A) The Lung Injury Score in the control group, SCS, and EVLP group (*, p <0.05). (B) Representative hematoxylin and eosin (H&E) stained slices of cold static preservation (SCS) donors. The black arrow indicates rupture of the pulmonary capillary epithelium, and the blue arrows indicate thickening of the alveolar walls. (C) Representative H&E-stained slices of EVLP donors. The red arrow indicates infiltration of inflammatory cells. (D) Representative H&E-stained slices of direct harvest donors (control). The black arrows indicate rupture and bleeding of pulmonary capillary epithelium. The error bars indicate standard deviation, significance analysis was performed by one-way analysis of variance (ANOVA). Please click here to view a larger version of this figure.
Discussion
This study introduces a cost-effective and modular design for a small animal EVLP platform and validation of the efficacy of this platform in a rat model of DCD. The findings indicate that EVLP can significantly improve the quality of lungs from deceased donors, thereby expanding the pool of potential donors for lung transplantation. The scarcity of donor lungs for transplantation remains a critical challenge. To address this issue, some countries have begun implementing DCD programs28. This approach offers a valuable strategy to increase the number of viable organ grafts. Notably, multiple studies have shown that long-term survival rates after liver transplantation using DCD organs are comparable to those using organs from DBD29,30. However, the utilization of lungs from DCD donors remains limited due to the extensive damage caused by prolonged ischemia31. Efficient evaluation and utilization of the limited lung resources obtained from DCD donors hold immense potential to alleviate the shortage of transplantable lungs. EVLP is a promising technique that can improve the quality of lungs and extend their preservation time before transplantation. To validate the effectiveness of EVLP compared to cold storage at 4 °C, we employed a rat model of circulatory death. We hypothesize that our perfusion technique can mitigate the damage caused by ischemia-reperfusion injury and enhance lung function following circulatory death. Our experimental results are consistent with recent findings reported by Wang et al.32 and Hasenauer et al.33.
Maintaining a consistent temperature throughout the perfusion circuit is crucial for optimal EVLP function. However, many existing EVLP systems lack reliable and adaptable temperature control mechanisms. Single heat exchange systems often require adjustments that alter the temperature in specific locations. This fluctuation in temperature can affect the composition of the perfusion fluid, disrupt oxygenation index measurements, and ultimately compromise the accuracy of experimental results. In order to overcome this difficulty, an EVLP system was created and fitted with three separate water bath heat exchangers that were placed in the reservoir, pulmonary artery perfusion zones, and organ chamber. Additionally, the system utilizes minimal piping to minimize heat loss and ensure precise temperature control within a range of 4-40 °C. This configuration allows for highly accurate temperature control, with measured temperatures in each circuit segment deviating no more than ± 0.2 °C from the set point. The design effectively meets the stringent temperature control requirements for EVLP experiments.
Small animal models, due to their smaller organs, are particularly susceptible to pulmonary edema and atelectasis34. These complications significantly hinder conducting lengthy perfusion experiments. To address this challenge and ensure optimal lung protection during perfusion, we adopted the Toronto approach, the current gold standard in clinical practice. This method involves a gradual increase in both perfusate flow rate and ventilation over a set time frame35. Perfusion was initiated at 10% of the target flow rate (20% of the cardiac output = 75 mL/min/250 g donor weight) and gradually increased over 1 h. Similarly, ventilation was initiated at a low rate (4 mL/kg), and the tidal volume was gradually increased to a maximum of 6 mL/kg over 10 min. The research also highlights the benefits of adding bovine serum albumin (BSA) to the perfusate. BSA supplementation elevates colloid osmotic pressure, thereby reducing tissue edema36. In small animal EVLP, two primary perfusion configurations exist based on atrial cannulation: closed-loop and open-loop. Closed-loop systems maintain a constant, low pressure within the atria, mimicking physiological conditions and facilitating accurate blood gas measurements. A study by Linacr et al. has demonstrated that maintaining a specific atrial pressure during perfusion can improve lung function and reduce edema34. However, pressure spikes can occur if atrial muscle obstructs the circuit. Additionally, tethering the atrium and the presence of air bubbles can compromise experimental results due to potential myocardial fiber damage and inconsistent pressure readings7. To overcome these limitations and achieve effective closed-loop perfusion, a specially designed atrial cannula was made. This cannula features a projecting brace at its tip to prevent obstruction by atrial muscle. Additionally, annular grooves along the cannula body facilitate secure ligature placement. Furthermore, the system incorporates bubble traps and pre-perfusion techniques to eliminate air embolism and maintain consistent perfusion pressure.
Small animal EVLP platforms are favored in scientific research for their numerous benefits: controlled animal conditions, cost-effectiveness, accommodation of large sample sizes, and low perfusion fluid consumption. Additionally, using a single small animal strain (e.g., rats in this study, but also guinea pigs and rabbits24,35,36) allows for meticulous control of both diet and experimental settings. The system’s versatility enables adaptation for all these species by adjusting breathing parameters and perfusion flow rate. Accompanying videos detail the animal model and EVLP procedures, providing researchers with a comprehensive understanding. By following these protocols, researchers can enhance experimental success, minimize animal suffering, and reduce overall animal use.
While the research has established an affordable, modular, and user-friendly EVLP system for small animals that surpasses commercial options in stability, perfusion quality, and adjustability, further investigation is necessary to optimize EVLP protocols, including perfusion and ventilation parameters, and identify the most effective lung-protective drugs. This user-friendly and efficient technology has enabled successful testing on many donor lungs leveraging infrastructure. Current research efforts are focused on the restoration and conservation of lungs from donors.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National National High-Level Hospital Clinical Research Funding and the Elite Medical Professionals project of China-Japan Friendship Hospital (Grant numbers[2022-NHLHCRF-LX-03-0303] and [ZRJY2021-GG07]).
Materials
| 3.0mm Medical aseptic tubing | Zhifang Pump industry Co., LTD, Hebei, China | 3.0mm | In the circulation of the blood, rather than blood vessels |
| Bovine serum albumin | Sigma-Aldrich Trading Co.Ltd, Shanghai, China | BSAV-RO | Enhance the colloid osmotic pressure of the perfusion |
| BP-100/102 Intravascular Blood Pressure Transducer | iWorx Systems, Inc, New Hampshire, USA | BP-100/102 | Records of pulmonary vascular pressure in rats |
| Cefazolin | Beijing psaitong Biotechnology Co., Ltd, Beijing, China | PHR1291 | Anti-bacterial infection |
| Deoxygenator | Dongguan Kewei Medical Instrument, Guangdong, China | No model specification | Replace Rat lung function Gas exchange in the blood |
| F500 General anesthesia machine for small animals | Beijing Yi Zejia Technology Co., LTD, Beijing, China | F500 | Administer gas anesthesia for the provision of anesthesia |
| Gas mixture (6% O2, 8% CO2, 86% N2) | Beijing Oriental Medical Gas Co., LTD, Beijing, China | No model specification | Maintain blood acid-base balance, simulates pulmonary vascular physiology |
| Heat exchanger | Changhong Precision Instrument Center, Shenzhen, China | DHC-05-B | Temperature control in the perfusion circuit |
| heparin | Beijing psaitong Biotechnology Co., Ltd, Beijing, China | H0200000 | Anti-micro thrombosis |
| Isoflurane | RWD Life Science Co.,Ltd, Shenzhen, China | R510-22 | Anesthetized rats |
| Isolation chamber | Hand-made of Shanghai Leighton Industrial Co., LTD, Shanghai, China | No model specification | Storage of rat lungs |
| i-STAT 1 Portable Clinical Analyzer | Abbott point of care, Inc, NJ, USA | 300G | The analysis of blood gas in perfusion |
| iWorx RA-834 Recorder | iWorx Systems, Inc, New Hampshire, USA | RA-834 | Pulmonary blood vessel pressure signal output |
| Multi-channel peristaltic pump LabMate | Zhifang Pump industry Co., LTD, Hebei, China | LabMate2 | Supplies energy for the process of perfusion circulation |
| PERFADEX Plus | XVIVO Perfusion, Gothenburg, Sweden | NA | The lung from the rat donor underwent prelavage; low-potassium solution |
| pulmonary cannula | Made of stainless steel | 304 stainless steel/SUS304 | 2.0/2.5 mm |
| Pulmonary vein cannula | Made of stainless steel | 304 stainless steel/SUS304 | 4.0mm |
| QAH-INF-1 assay kit | Innopsys, Parcd'Activités Activestre | ||
| R415 Small animal ventilator | RWD Life Science Co.,Ltd, Shenzhen, China | R415 | The ventilation of the rat lung |
| Reservoir | Shanghai Leighton Industrial Co., LTD, Shanghai, China | Capacity 1 L | Perfusion storage |
| SOLU-MEDROL | Beijing psaitong Biotechnology Co., Ltd, Beijing, China | M0639 | NA |
| Temperature Sensor (TM-100) | iWorx Systems, Inc, New Hampshire, USA | TM-100 | Record lung perfusion temperature |
References
- Domínguez-Gil, B., et al. Expanding controlled donation after the circulatory determination of death: Statement from an international collaborative. Intensive Care Med. 47 (3), 265-281 (2021).
- Valapour, M., et al. Optn/srtr 2020 annual data report: Lung. Am J Transplant. 22 Suppl 2, 438-518 (2022).
- Holm, A. M., Courtwright, A., Olland, A., Zuckermann, A., Van Raemdonck, D. ISHLT position paper on thoracic organ transplantation in controlled donation after circulatory determination of death (cdcd). J Heart Lung Transplant. 41 (6), 671-677 (2022).
- Chiu, S., Mills, S. E. A., Bharat, A. Expanding the lung donor pool and improving outcomes: Ex vivo lung perfusion. JAMA Surg. 154 (12), 1151 (2019).
- Nakajima, D., Date, H. Ex vivo lung perfusion in lung transplantation. Gen Thorac Cardiovasc Surg. 69 (4), 625-630 (2021).
- Furukawa, M., et al. Lung transplantation from donation after circulatory death, evolution, and current status in the United States. Clin Transplant. 37 (3), e14884 (2023).
- Valenza, F., et al. Ex vivo lung perfusion to improve donor lung function and increase the number of organs available for transplantation. Transpl Int. 27 (6), 553-561 (2014).
- Divithotawela, C., et al. Long-term outcomes of lung transplant with ex vivo lung perfusion. JAMA Surg. 154 (12), 1143-1150 (2019).
- Egan, T. M., et al. Donation after circulatory death donors in lung transplantation. J Thorac Dis. 13 (11), 6536-6549 (2021).
- Kanou, T., et al. Cell-free DNA in human ex vivo lung perfusate as a potential biomarker to predict the risk of primary graft dysfunction in lung transplantation. J Thorac Cardiovasc Surg. 162 (2), 490-499.e492 (2021).
- Loor, G., et al. Portable normothermic ex vivo lung perfusion, ventilation, and functional assessment with the organ care system on donor lung use for transplantation from extended-criteria donors (expand): A single-arm, pivotal trial. Lancet Respir Med. 7 (11), 975-984 (2019).
- Cypel, M., et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 364 (15), 1431-1440 (2011).
- Yeung, J. C., et al. Outcomes after transplantation of lungs preserved for more than 12 h: A retrospective study. Lancet Respir Med. 5 (2), 119-124 (2017).
- Cypel, M., Keshavjee, S. The clinical potential of ex vivo lung perfusion. Expert Rev Respir Med. 6 (1), 27-35 (2012).
- Steen, S., et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 83 (6), 2191-2194 (2007).
- Lindstedt, S., et al. Comparative outcome of double lung transplantation using conventional donor lungs and non-acceptable donor lungs reconditioned ex vivo. Interact Cardiovasc Thorac Surg. 12 (2), 162-165 (2011).
- Wallinder, A., et al. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: A case-control study. Eur J Cardiothorac Surg. 45 (1), 44-45 (2014).
- Cypel, M., et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 27 (12), 1319-1325 (2008).
- Frank, J. A., et al. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol. 293 (1), L52-L59 (2007).
- Inci, I., et al. Fibrinolytic treatment improves the quality of lungs retrieved from non-heart-beating donors. J Heart Lung Transplant. 26 (10), 1054-1060 (2007).
- Ohsumi, A., et al. A method for translational rat ex vivo lung perfusion experimentation. Am J Physiol Lung Cell Mol Physiol. 319 (1), L61-L70 (2020).
- Nelson, K., et al. Method of isolated ex vivo lung perfusion in a rat model: Lessons learned from developing a rat evlp program. J Vis Exp. (96), e52309 (2015).
- Bassani, G. A., et al. Ex vivo lung perfusion in the rat: Detailed procedure and videos. PLoS One. 11 (12), e0167898 (2016).
- Cleveland, W. J., et al. Design and implementation of a rat ex vivo lung perfusion model. J Vis Exp. (195), e64740 (2023).
- Oliveira, P., Yamanashi, K., Wang, A., Cypel, M. Establishment of an ex vivo lung perfusion rat model for translational insights in lung transplantation. J Vis Exp. 10 (199), e65981 (2023).
- Nelson, K., et al. Animal models of ex vivo lung perfusion as a platform for transplantation research. World J Exp Med. 4 (2), 7-15 (2014).
- Van Zanden, J. E., Leuvenink, H. G. D., Verschuuren, E. a. M., Erasmus, M. E., Hottenrott, M. C. A translational rat model for ex vivo lung perfusion of pre-injured lungs after brain death. PLoS One. 16 (12), e0260705 (2021).
- Choi, K., et al. Early national trends of lung allograft use during donation after circulatory death heart procurement in the United States. JTCVS Open. 16, 1020-1028 (2023).
- Krutsinger, D., et al. Lung transplantation from donation after cardiocirculatory death: A systematic review and meta-analysis. J Heart Lung Transplant. 34 (5), 675-684 (2015).
- Sabashnikov, A., et al. Long-term results after lung transplantation using organs from circulatory death donors: A propensity score-matched analysis. Eur J Cardiothorac Surg. 49 (1), 46-53 (2016).
- Zhao, J., et al. The low utilization rate of donor lungs in china: A single-center experience. Ann Transplant. 26, e931409 (2021).
- Wang, W., Qian, J., Zhu, M., Wang, Y., Pan, Y. Normothermic ex vivo lung perfusion outperforms conventional cold preservation in a deceased rat lung. Ann Transl Med. 10 (2), 99 (2022).
- Hasenauer, A., et al. Effects of cold or warm ischemia and ex-vivo lung perfusion on the release of damage associated molecular patterns and inflammatory cytokines in experimental lung transplantation. J Heart Lung Transplant. 40 (9), 905-916 (2021).
- Linacre, V., et al. Importance of left atrial pressure during ex vivo lung perfusion. J Heart Lung Transplant. 35 (6), 808-814 (2016).
- Pêgo-Fernandes, P. M., et al. Experimental model of isolated lung perfusion in rats: First brazilian experience using the il-2 isolated perfused rat or guinea pig lung system. Transplant Proc. 42 (2), 444-447 (2010).
- Pacheco-Baltazar, A., Arreola-Ramírez, J. L., Alquicira-Mireles, J., Segura-Medina, P. Isolated lung perfusion system in the rabbit model. J Vis Exp. (173), e62734 (2021).

.
