Automatically Generated
An Affordable and Efficient “Homemade” Platform for Drosophila Behavioral Studies, and an Accompanying Protocol for Larval Mitochondrial Respirometry
Summary
We provide protocols for anyone with a “maker culture” mind to start building a flylab for quantitative analysis of a myriad of behavioral parameters in Drosophila melanogaster, by 3D-printing many of the necessary pieces of equipment. We also describe a high resolution respirometry protocol using larvae to combine behavioral and mitochondrial metabolism data.
Abstract
The usefulness of Drosophila as a model organism for the study of human diseases, behaviors and basic biology is unquestionable. Although practical, Drosophila research lacks popularity in developing countries, possibly due to the misinformed idea that establishing a lab and performing relevant experiments with such tiny insects is difficult and requires expensive, specialized apparatuses. Here, we describe how to build an affordable flylab to quantitatively analyze a myriad of behavioral parameters in D. melanogaster, by 3D-printing many of the necessary pieces of equipment. We provide protocols to build in-house vial racks, courtship arenas, apparatuses for locomotor assays, etc., to be used for general fly maintenance and to perform behavioral experiments using adult flies and larvae. We also provide protocols on how to use more sophisticated systems, such as a high resolution oxygraph, to measure mitochondrial oxygen consumption in larval samples, and show its association with behavioral changes in the larvae upon the xenotopic expression of the mitochondrial alternative oxidase (AOX). AOX increases larval activity and mitochondrial leak respiration, and accelerates development at low temperatures, which is consistent with a thermogenic role for the enzyme. We hope these protocols will inspire researchers, especially from developing countries, to use Drosophila to easily combine behavior and mitochondrial metabolism data, which may lead to information on genes and/or environmental conditions that may also regulate human physiology and disease states.
Introduction
Drosophila melanogaster was introduced to the scientific community as a potentially powerful model organism more than 100 years ago. That potential has been firmly validated in several areas of the biological and biomedical sciences, such as genetics, evolution, developmental biology, neurobiology, and molecular and cell biology. As a result, six Nobel Prizes in Medicine or Physiology have been awarded to ten Drosophila researchers who have substantially contributed to our understanding of heredity, mutagenesis, innate immunity, circadian rhythms, olfaction and development1. Perhaps more importantly, D. melanogaster has not ceased to provide us with new models of human biology and diseases, as a quick search on PubMed reveals almost 600 publications in the last 5 years, using the search term "drosophila model" (2, as of February, 2021). In the US, where Drosophila is a wide spread model organism in the biomedical community, about 2.2% of all R01 research awards granted by the NIH in 2015 were allocated to Drosophila researchers3. In Brazil, on the other hand, a search for currently funded projects on the website of the Sao Paulo Research Foundation (FAPESP), the most important funding agency for research in all scientific areas in the state of Sao Paulo, showed only 24 grants and fellowships with Drosophila as the main subject of study4. Considering all 13205 projects currently funded by FAPESP (5, as of February, 2021), those 24 Drosophila projects represent a ratio of less than 0.2% of the total projects, which is nearly 12 fold lower than that of the NIH. If we remove the funded projects that aim at studying Drosophila from an ecological and/or evolutionary point of view, and assume that the remaining projects use this organism as a model for understanding human biological processes in health and disease, that ratio decreases to a shocking ~0.1%.
In fact, a proper investigation is warranted to reveal the reasons why Drosophila research in Brazil/Sao Paulo does not appear to be as significant in number of funded projects. Culturing Drosophila is not expensive6,7,8 and is relatively simple, as unlike vertebrates, no permission from a bioethical committee is necessary for experimentation9,10. An approval to work with genetically modified fly lines is, however, required in Brazil11, adding a layer of bureaucracy inherent to all work involving genetically modified organisms. However, this would likely not prevent interested researchers from initiating a flylab. We speculate that misinformation about the power of the model, and about the expected high costs associated with setting up a flylab and performing meaningful experiments are important factors in this decision. As for most science equipment and supplies, the appropriate apparatuses to perform general fly maintenance and behavioral analyses must be imported into Brazil from North America, Europe and/or elsewhere, which is an expensive and extremely time consuming process12,13.
Recently, an alternative to importing specialized apparatuses has emerged as 3D printers have become more affordable and accessible to any person, including Drosophila researchers in developing countries. The 3D-printing technology has been widely used in the last 10 years by members of the "maker culture", which is based on the idea of self-sufficiency over exclusively relying on company manufactured products14. Such an idea has always been present in academic research laboratories around the globe, so it is not surprising that 3D printers have become standard lab equipment in many places15,16. For a number of years, we have been 3D-printing fly vial racks, mating arenas, climbing apparatus, among other devices, for a fraction of the cost of brand-named equivalents. The reduced costs of printing and assembling homemade lab equipment is classically represented by the FlyPi, which can be built for less than €100.00 and serves as a light and fluorescence microscope able to use sophisticated opto- and thermogenetic stimulation of the genetically tractable zebrafish, Drosophila and nematodes15. Here, we provide a series of protocols for anyone interested in becoming a Drosophila researcher (or in expanding his/her own existing flylab) to 3D-print many of the necessary material. By investing time and developing a little expertise, the reader will even be able to optimize the protocols presented here to print apparatuses better adapted to his/her own research needs.
However, a flylab is not a place for "cheap" equipment only, especially when one intends to associate behavioral analyses with underlying metabolic phenomena. We have also been interested in the roles of mitochondria in the modulation of Drosophila behavioral patterns, as these organelles are responsible for the bulk production of ATP in most tissues through several metabolic pathways whose products converge to oxidative phosphorylation (OXPHOS). Analyzing mitochondrial oxygen consumption as a way to understand mitochondrial metabolism does require an oxygraph, which is a more sophisticated piece of equipment that unfortunately cannot yet be 3D-printed. Because OXPHOS impacts practically all cellular processes since it depends on a series of exergonic redox reactions that occur in the cell17,18, oxygen consumption rates based on the oxidizable substrate provided to mitochondria may help reveal whether the organelle´s functioning is cause or consequence of a particular behavior. Therefore, we also provide here a protocol for measuring mitochondrial oxygen consumption in larva samples, as we realize the vast majority of published protocols are focused on analyzing adult samples. We show that changes in mitochondrial respiration, induced by the transgenic expression of the Ciona intestinalis alternative oxidase (AOX), leads to increased larval mobility under cold stress. This is most likely due to thermogenesis, since AOX is a non-proton pumping terminal oxidase that can bypass the activity of OXPHOS complexes III and IV (CIII and CIV), without contributing to the mitochondrial membrane potential (ΔΨm) and ATP production19,20,21. No insect, including Drosophila, or vertebrate naturally possesses AOX21,22,23, but its expression in a myriad of model systems24,25,26,27,28,29 has been successful to show its therapeutic potential for conditions of general mitochondrial respiratory stress, especially when caused by CIII and/or CIV overload. AOX confers resistance to toxic levels of antimycin A24 and cyanide24,25, and mitigates diverse phenotypes related to mitochondrial disfunction24,25,30,31,32. The fact that AOX expression changes larval behavior and mitochondrial function justifies more in-depth studies of this enzyme's roles in the metabolism and physiology of metazoan cells and tissues33,34.
We hope that with this article we can help raise awareness within the scientific community of developing countries such as Brazil that using the excellent genetic toolset that D. melanogaster presents, in combination with efficient and affordable homemade apparatuses for behavioral analyses, can generate relatively fast basic research data on interesting biological processes with significant translational impact, supporting future therapeutic studies in clinical research. Developing such communal ideals would greatly benefit Drosophilists, medical researchers, and the biological and biomedical sciences. Most importantly, it would benefit society in general, as public funding could be applied more translationally to understand and treat human diseases.
The protocols we provide here for 3D printing the apparatuses for a flylab were designed for use with the RepRap 3D printer, based on the Prusa I3 DIY model available at35. We use the 1.75-mm white polylactic acid (PLA) filament (SUNLU) as raw material for printing, the Tinkercad platform36 for model design, and the Repetier-Host software37 for STL to G-Code conversion, a necessary step to provide coordinates to the printer. Further optimization of the protocols is required should the reader want to use alternative equipment, materials and software.
Protocol
1. 3D model design
NOTE: The workflow for 3D printing has three basic steps: (1) 3D modeling; (2) importing the model into the slicing software; and (3) selecting the correct filament, configuring the printer, and finally, printing. A basic protocol for modeling a small fly vial rack/tray is shown below; this rack is to be used with standard fly vials, which have approximately 2.5 cm in diameter and 9.8 cm in height. For new model designs, the tools provided by the Tinkercad software allow the easy handling of three-dimensional structures, by creating pieces of different shapes, sizes and thickness, according to one's own needs. For Drosophilists venturing for the first time into the realm of 3D printing, following the protocols below, even with all their details, may still be challenging, so we strongly recommend becoming acquainted with the software for best results.
- Sign in to Tinkercad online38 (Figure 1A). Prior registration with personal information is required to access the platform, free of charge.
- Click on Create a new Project to begin a new design, and rename the Project accordingly on the upper right corner of the window. Press Enter to be directed to the project´s workplane (Figure 1B).
- Verify if the workplane has the correct dimensions of 200 mm x 200 mm, by clicking with the mouse´s left button on Edit Grid at the lower right corner (red square in Figure 1B). In the popup window (Figure 1C), make sure that the "Units" are millimeters, and the "Presets" are default. Enter 200.00 in the "Width" and in the "Length" fields, and click on Update Grid to save the changes.
- Verify if the Snap Grid is set to 1.0 mm (red square in Figure 1B). If it is not, click on the dropdown menu and select 1.0 mm.
- Under the Basic Shapes menu on the right (blue square 2 in Figure 1B), select a solid box and drag it to the center of the workplane.
- Click anywhere on the box in the workplane with the mouse´s left button to see its edges and vertices. Click on any vertex (which will then turn red) to show the box dimensions (red squares in Figure 1D). Click with the mouse´s left button on each dimension and type 130 mm for length (L), 130 mm for width (W) and 40 mm for height (H). Recenter the box by dragging it to the middle of the workplane.
- Click on the tool Ruler on the upper right corner of the screen (red square 1 in Figure 1E). Immediately click on the lower left vertex of the box, as indicated in Figure 1E (red square 2), to set the initial point (x = 0, y = 0, z = 0) of a tridimensional Cartesian coordinate system. Note that the distance between the selected vertex and the coordinate initial point will now show (red squares in Figure 1F), where "A", "B" and "C" represent the distance to the x, y and z axes (which should be zero in this case), respectively.
- Next, select an empty (hole) box from the Basic Shapes menu on the right (blue square 2 in Figure 1B) and drag it to the workplane. Set its dimensions to 30 (L) x 30 (W) x 40 (H) mm, and elevate it 2 mm from the workplane, by typing "2.00" in the textbox next to the green arrowhead on the lower right corner of the empty box (red square in Figure 1G). Position the empty box inside the solid box 2 mm away from the x,y coordinate initial point, by typing "2.00" in the textboxes next to the green arrows on the lower left corner of the box (red squares in Figure 1H; compare with Figure 1G).
- With the empty box still selected, press the CtrL+D keys on the keyboard to deploy the "duplicate" command and create a new empty box of the exact same dimensions. Position the new empty box inside the solid box, next to the first empty box, by typing "34.00" in the textbox next to the green arrow along the y axis, and "2.00" in the textboxes next to the two remaining green arrows (red squares in Figure 1I).
- Repeat this step, adjusting for the correct distances from the coordinate initial point, until the entire solid box is filled with empty boxes spaced 2 mm apart from each other (Figure 1J).
- Select all the boxes (solid and empty ones) by clicking with the mouse´s left button and dragging to the entire area. Press the CtrL+G keys on the keyboard to deploy the "group" command and create a single box with 16 empty spaces for fly vials (Figure 1K). This is the final design of the vial rack.
- Click on Export on the upper right corner of the Tinkercad window. On the window box displayed (Figure 1L), select Everything in the design next to Include, and .STL under For 3D Print as the file type. Choose a proper name for the design file and save it in an appropriate place in the computer.
2. 3D printing
NOTE: In this section, we provide instructions on how to use the STL file created in Step 1 and convert it to the G-Code file containing the printing instructions to the 3D printer. This is the slicing process, for which we use the Repetier-Host software.
- Download Supplemental File 1 and save it on an appropriate place in the computer. This is a .rcp file containing the printer configurations to be used below. To get more information on the .rcp file type, please visit39.
- Open the Repetier-Host software, which should already be installed the computer, following instructions from37. Press the CtrL+O keys on the keyboard to open the STL file on the computer created in Protocol 1.
- Once opened, click on the designed vial rack and press the R key on the keyboard to open the editing menu on the right side of the screen (red square 1 in Figure 2A). Centralize the object on the printing table by clicking on the Center Object button, indicated with red square 2 in Figure 2A, on the Object Placement tab.
- Click on the Slicer tab (red square 1 in Figure 2B) next to Object Placement tab, and then click on the Configuration button (red square 2 in Figure 2B) below. Note that a new window on the left will open where the printer parameters such as velocity, layer thickness and holders can be defined (see more details in the Discussion below).
- Click on the Import button (red square 3 in Figure 2E), select Supplemental File 1 from the files, and press Enter. Note that this .rcp file (downloaded in step 2.1) provides the parameters for the automatic configuration of the printer we have optimized for this vial rack.
- To finish configuring the printing parameters, select None for Support Type on the menu on the right (red square 4 in Figure 2E), as printing of this piece does not require a support to prevent bending or other deformities. In Infill Density (red square 5 in Figure 2E), choose 20% to create a solid structure (see more details about these parameters in the Discussion below).
- Click on Slice with CuraEngine on the upper right corner of the screen to run the slicing program and generate the G-Code, which has the information necessary for the printer to print the piece. Note that under the Print Preview tab on the menu on the right (next to the Slicer tab), information about the time and amount of material required for the printing job to be completed will then show (red square 1 in Figure 2F).
- Click on Save for SD Print to save the G-Code file in a SD card (red square 2 in Figure 2F). Note that the G-Code contains the 3D coordinates of the designed piece, sliced into layers, for proper function of the printer.
- Insert the SD card in the RepRap 3D printer, and then follow the information displayed on the printer screen to select printing from the SD card.
- Select the G-Code file of the vial rack. Note that the printer will automatically warm up and start printing the designed piece, which should take several hours. The rack (Figure 3A) should be ready for use immediately after the printing job is completed.
3. Behavioral analysis apparatuses
NOTE: The steps described in Protocols 1 and 2 can be repeated with appropriate adjustments to print several of the pieces of lab equipment needed. However, we realize that designing new pieces may be challenging and time-consuming for beginner users of Tinkercad, so instead of providing step-by-step protocols on how to design all models, we are making available for download several design models we created as STL files (see Supplemental Files 2-11).
- Download Supplemental File 2 for a model of a small funnel (Figure 3B), routinely used in flylabs to help transfer adult flies to new vials or bottles by avoiding flies crawling up the inside walls of these containers to escape.
- Download Supplemental File 3 for a tapping mat support (Figure 3C), which can hold an ethylene-vinyl acetate foam or a thick cotton mat onto which glass vials or bottles can be tapped when one is tipping flies into new containers with freshly made food.
- Download Supplemental File 4 and Supplemental File 5 for the model of a camera stand that we named Stalker (Figure 3D).
NOTE: The apparatus allows any camera (professional, webcams, cellphones, etc.) to be positioned on top of a base where a Petri dish containing larvae or adult flies can be imaged or video recorded. Stalker allows the imaging of animal behavior that takes place horizontally, always at the same distance from the Petri dish, which avoids introducing variability into the experimental measurements if the recordings must be made on different days, for example, or if the camera needs to be used for another purpose in between recordings. The apparatus is conveniently modular and can be easily assembled after printing the base and the top from Supplemental File 4, and the sides from Supplemental File 5. The 1 cm2 squares at the base, which can be highlighted using a permanent marker, help track distance travelled by individual animals. Print the apparatus (at least the base) using white filament, so that there is enough contrast between the background and the animals for the tracking software to identify each fly. - Download Supplemental File 6 for the printable design of the Fly Motel (Figure 3E), which has ten courtship and mating arenas (rooms) organized in a manner to facilitate video recordings of ten individual mating pairs at a time. Note that the Fly Motel is based on the device published in40, where detailed explanation for its use in behavioral studies is found. In addition to the 3D-printed parts, the apparatus requires 12 screws (3 x 8 mm) for fixing the upper part to the lower one to stabilize the assembled device, an acrylic plate (60 x 60 x 3 mm), and a zip tight. Because the structure of the Fly Motel is more complex, we also provide an instructional video (Supplemental File 7) of how to assemble it correctly, given that all required pieces and a screwdriver are available.
- Download Supplemental File 8 for the model of a T-Maze (Figure 3F), which is used for memory assays using adult flies. A detailed explanation of how the T-Maze is used to make flies associate repulsive odor stimuli with their phototropic behavior is found in the original publication41. The apparatus also makes use of two translucent 15 mL conical tubes, which are commonly found in any lab and are attached to the 2 cm-wide circular openings in each side of the central piece. Note that the printing of the T-Maze requires a support (see more details in the legend to Figure 2). Select "Everywhere" for Support Type (red square 4 in Figure 2E) after following steps 2.1-2.6 above.
- Download Supplemental File 9 and Supplemental File 10 for the design of the printable parts of our version of the apparatus for rapid iterative negative geotaxis (RING) assays (Figure 3G), used to perform climbing assays with adult flies of several genotypes or environmental conditions simultaneously, generating results in a more standardized and higher-throughput fashion42. The RING apparatus is also modular, and in addition to the 3D-printed parts, it requires other pieces that can be easily purchased online or in a hardware store at low cost: two Φ8 x 300 mm rectified shafts, four Φ8 mm linear bearings, rubber bands (or pieces of a string), a 240 x 60 x 20 mm piece of wood for the base, and eight wood screws (8 mm) to fix the printed parts to the wood base. Download Supplemental File 11 for instructions on how to assemble the device once all parts are printed or purchased. A detailed explanation of how to use the RING apparatus is found in the original publication42.
4. Larval mobility assay
NOTE: We have optimized this protocol, originally based on Nichols et al.42, to study the effects of AOX expression on Drosophila development under cold stress. The lines 3xtubAOX25 and w1118, used as examples of AOX-expressing and control larvae, respectively, were cultured on standard diet24 at 12 °C, according to Saari et al.34. We recommend this protocol to analyze the mobility of larval samples of any genetic condition, cultured under any environmental condition of interest.
- Prepare 2% agar plates by boiling the equivalent amount of agar in deionized water, pouring into Φ90 X 15 mm Petri dishes, and allowing them to solidify at room temperature. For plates with a final volume of 20 mL each, use 0.4 g of agar.
- Carefully collect wandering L3 larvae from the side of the culture vials/flasks using a pair of round tip forceps or a brush, and place the individuals in a Φ90 x 15 mm Petri dish with deionized water for less than 10 seconds to rinse food particles attached to their body.
- Transfer individual larva to dishes with agarose and wait 5 minutes for the animals to acclimate.
- Position the dishes containing the individual larva on top of a graph paper (0.2 cm2 grid), and count the number of lines crossed by the animal for 1 minute, as it moves on top of the agar. Each line crossed represent a distance of 2 mm. Repeat the procedure with the same individual 10-15 times to obtain technical replicates.
- Repeat step 4.4 with at least 8-10 individuals from different tubes/flasks, cultured at different times to obtain biological replicates of the same line.
- Repeat steps 4.4 and 4.5 to obtain data for the other fly line (or for as many lines as one intends to analyze).
- The same individual larvae used in steps 4.4-4.6 may be used to obtain additional data on body movement by placing the agar dishes with an individual larva under a stereomicroscope and counting the number of peristaltic contractions of the body wall for 1 minute. Obtain technical and biological replicates for an estimate of average mobility among the fly lines analyzed.
- Apply the statistical test to calculate the probability of the values of these larval mobility parameters being distinct among the lines of interest. Because we are comparing here data from only two lines, a Student's t test may be applied.
5. Mitochondrial respirometry using larval homogenates
NOTE: The following protocol was optimized to measure mitochondrial oxygen consumption from larval homogenates of the AOX-expressing line 3xtubAOX and the control w1118, cultured at 12°C, but we also recommend it to be used for larval samples of any genetic and environmental conditions. We realize that conducting such experiments should not be included as an "affordable" goal for a "home-made" flylab, unlike all other protocols we provide in this article, as a considerable initial investment must be made for a lab to acquire a high resolution oxygraph. The protocol is to be used with the Oxygraph-2k (O2k) and the DatLab software from Oroboros Instruments, so further optimization is required should the reader want to use an alternative equipment.
- Turn on the O2k and start the DatLab software in the computer connected to the oxygraph. The magnetic bars inside the assay chambers should start stirring automatically. Remove the ethanol storage solution from the chambers.
- Wash the chambers at least 3 times with 100% ethanol and 3 times with ultrapure water.
- In DatLab, the window O2k Control will open automatically. In Block temperature [°C], enter 12 °C (or the preferred temperature in the range of 2-47 °C) and press OK.
- A second window, Edit Experiment, will then open automatically. Enter sample names in the Sample fields, according to what will be added in chambers A and B, and press Save.
- Add 1800 µL of the assay buffer (120 mM KCl, 5 mM KH2PO4, 3 mM Hepes, 1 mM EGTA, 1 mM MgCl2, 2% BSA, pH 7.4) in each chamber and close them partially, still allowing oxygen exchange with the outside air. Allow the oxygen concentration and oxygen flux signals to stabilize, showing minimal fluctuations for at least 10 min. This step will provide the calibration of the equipment with the outside air on the day of the experiment (see steps 6.3 and 6.4 below for further details on experimental calibrations).
- During the air calibration step, initiate sample preparations by carefully collecting from the culture tubes/flasks 20 wandering larvae of the appropriate genotype, using a pair of forceps or a small paintbrush.
- Rinse each larva quickly but thoroughly with deionized water or 1x PBS (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4 and 2 mM KH2PO4) and transfer them to a 1 mL glass homogenizer on ice, containing 500 µL of ice-cold isolation buffer (250 mM sucrose, 5 mM Tris HCl, 2 mM EGTA, pH 7.4)
- Homogenize the whole larvae with 5 strokes and pour the homogenate into a 1.5 mL microcentrifuge tube on ice.
- Add 300 µL of isolation buffer to the glass homogenizer, macerate the remaining larval tissues further with 3 strokes, and pour the homogenate again into the same microcentrifuge tube on ice.
- Add 200 µL of isolation buffer to the glass homogenizer, macerate the residual larval tissues further with 2 strokes, and pour the homogenate again into the same microcentrifuge tube on ice.
- Mix the final homogenate (~1 mL) by inverting the tube twice gently and keep it on ice until the second sample is processed.
- Repeat steps 5.6-5.11 to obtain the larval homogenate of the other genotype.
- Open the oxygraph chambers A and B, and transfer 200 µL of each homogenate to the assay buffer in each chamber, which must be exactly at 12 °C at this point. Close the chambers completely and allow the oxygen concentration and oxygen flux signals to stabilize for approximately 10 min.
- Initiate oxygen consumption measurements by adding to each chamber 5 µL of a solution of 2 M pyruvate, 2 M proline (final concentration in the chambers = 5 mM each) and 7.5 µL of a solution of 0.4 M malate (1.5 mM). Allow at least 5 minutes for signal stabilization. Note that at this point, the mitochondria will be charged with oxidizable substrates to initiate the tricarboxylic acid (TCA) cycle reactions. Any increase in the oxygen consumption signal may be due to the functions of uncoupling proteins (or due to other uncoupling phenomena), and can be used to calculate general uncoupled respiration (also referred to as Leak respiration in the absence of adenylates, LN– see Representative Results for details).
- Add to each chamber 4 µL of a solution of 0.5 M ADP (1 mM) and allow at least 5 minutes for signal stabilization. A significant increase in the oxygen consumption signal is usually immediately observed, representing the oxidative phosphorylative (OXPHOS) respiration. The great majority of this OXPHOS respiration is driven by complex I (CI).
- Add to each chamber 2 µL of a solution of 0.05 M antimycin A (0.05 mM) to inhibit CIII, and allow at least 5 minutes for signal stabilization. A decrease in the oxygen consumption signal down to the basal levels seen before the addition of pyruvate, proline and malate should be observed for the w1118 control sample. Mitochondrial respiration from AOX-expressing larvae will be partially resistant to antimycin-A, as the electrons can now be directed to AOX. About 40% of the total OXPHOS respiration should remain after CIII inhibition, which should be all supported solely by AOX.
- Add to each chamber 4 µL of a solution of 0.1 M propyl-gallate (0.2 mM) to inhibit AOX, and allow at least 15 minutes for signal stabilization. The oxygen consumption levels in the AOX-expressing larval samples should now decrease to the basal levels seen before the addition of pyruvate, proline and malate. This decrease proves that the antimycin-A resistant respiration observed is due to AOX function.
- Add to each chamber 1 µL 0.01 M rotenone (0.005 mM) to inhibit CI, and allow at least 5 minutes for signal stabilization to obtain the oxygen consumption signal of the sample independent of mitochondrial respiration. This signal is usually as low as that observed before the addition of pyruvate, proline and malate.
- Save the experiment and close the DatLab software.
6. Mitochondrial respirometry data processing
NOTE: Oxygen consumption values are obtained as an average of the oxygen flux signals in a determined period of time and are expressed as pmol O2 consumed per second per mg total protein in the sample. The values are first referenced against the maximal oxygen concentration available in the assay buffer on the day of the experiment, based on the experimental temperature (referred to as air saturation), and the minimal oxygen concentration, which is determined previously in each chamber by the addition of Na2S2O4 to the assay buffer (see 43 for the manufacturer's guidelines to obtain zero oxygen calibration). The values are also normalized by the amount of total protein in the larval homogenates added to the assay buffer of each chamber.
- Determine total protein concentration of each sample using the Bradford method44. Reopen the saved experiment on the DatLab software, press Experiment at the top menu, and then Edit. In the opened window, select mg in the Unit section and in the Amount section enter the protein amount contained in the 200 µL of the sample added in each chamber. The concentration will be automatically calculated by the software, considering the total chamber volume of 2 mL. Press the Save button.
- Press Graph in the top menu, and then Select plots. In the opened window, select Flux per mass for both graphs (1 and 2, which refer to chambers A and B, respectively). Press Ok. The experimental outcome is now normalized by the protein concentration of the samples (pmol O2/s/mg total protein).
- On the upper right corner of each graph (1 and 2) of the main experimental outcome page, click on O2 Concentration. Along the x axis, identify a time range prior to addition of the samples in the chambers, in which the oxygen concentration and flux signals are very stable.
- Press and hold the Shift key on the computer's keyboard, click with the left mouse button on the initial time selected, drag the cursor along the time axis to select the desired region, and release the mouse button. Do this procedure for each of the graphs individually.
- Double-click on the blue bars of the selected areas at the bottom of the graphs, and type "air" to indicate that these are the selected regions used to calculate the oxygen air saturation.
- Click on Calibration on the top menu bar, and select A: Oxygen, O2 to calibrate chamber A. In the opened window, verify that O2 Calib is selected (yellow color).
- For the Zero calibration, click on Copy from file and choose the file with the previously performed zero oxygen calibration (see 43 for the manufacturer’s guidelines).
- For Air calibration, select air in the Select Mark column. Click on Calibrate and copy to clipboard.
- Click on Calibration on the top menu bar, select B: Oxygen, O2 and repeat step 6.4 to calibrate chamber B.
- On the upper right corner of each graph (1 and 2) of the main experimental outcome page, click on O2 flux per mass. Select desired stable regions of the oxygen consumption signal by holding the Shift key on the keyboard, clicking with the left mouse button, and dragging the cursor along the time axis. The stable regions selected between additions of pyruvate/proline/malate and ADP represents the LN; between ADP and antimycin A, OXPHOS; between antimycin A and propyl gallate (upon CIII inhibition), antimycin A-resistant respiration; between propyl gallate and rotenone (upon CIII+AOX inhibition), residual respiration; after rotenone (upon CI+CIII+AOX inhibition), residual non-mitochondrial respiration. Double-click on the red bars of the selected areas at the bottom of the graphs, and enter appropriate labels.
- Click on Marks on the top menu bar, and then Statistics. In the Show tab of the opened window, uncheck all options except O2 flux per mass. In the Select tab of the same window, select chamber A to obtain the respiration data for the first sample. Click on Copy to clipboard and paste the data into a spreadsheet. Repeat the procedure to obtain the data for the second sample in chamber B.
- In a spreadsheet, subtract all respiration values by the residual non-mitochondrial respiration (the data after rotenone addition). Calculate averages from multiple experimental replicates and plot the data as preferred.
Representative Results
By following the steps in Protocols 1 and 2, one should be able to design a simple fly vial rack, and run the model STL file through the slicing program to generate coordinates for the 3D printer. Figure 3A shows a printed unit of the model next to its design. We also hope step 1 can provide the basic skills for one to use the basic shapes available in the Tinkercad platform to create useful apparatuses for the lab. Developing these skills, however, may require constant practice and frequent consultations of the Tinkercad help center45. In Protocol 3, we provide our own designs for apparatuses that we routinely use for general fly maintenance and behavioral studies with adults or larvae. By following Protocol 2, a researcher should be able to use the STL files provided as Supplemental Files 2-6 and 8-10 in Protocol 3 to print tapping mat supports, funnels, Stalkers, Fly Motels, T-Mazes and RING apparatuses. Figure 3B-G shows side-by-side images of the model designs and of the actual printed and assembled apparatuses. It is important to mention that each printer may require minimal adjustments to the printing settings for optimal function; our Supplemental File 1, however, appears to provide a robust initial set of instructions for RepRap 3D printers to print the abovementioned devices.
In addition to providing instructions on how to create and 3D-print apparatuses that can be used for behavioral analyses in Drosophila, we also provide a protocol to perform assays and obtain behavioral measurements using larvae (Protocol 4) and a protocol for high resolution respirometry using larval homogenates (Protocols 5 and 6) to investigate mitochondrial metabolism. The mobility assays provided can easily reveal the impact on larval behavior at cold temperatures, when the fly OXPHOS is directly altered by ubiquitous expression of AOX. AOX-expressing larvae can cover distances ~70% longer than control larvae at the stressful temperature of 12 °C (Figure 4A), apparently due to the ~40% higher number of peristaltic movements of the body wall (Figure 4B). We can also easily associate this change in behavior with changes in mitochondrial function. Figure 5A shows the traces of oxygen consumption as a representative outcome of an experiment using larval homogenates. It is evident that the mitochondria of AOX-expressing larvae have antimycin A-resistant respiration, which is sensitive to the AOX inhibitor propyl-gallate (green arrow in Figure 5A, and Figure 5B), and consistent with previously published data using adult flies and with the expected function of AOX24. Quantification of the different oxygen consumption states also indicates that LN respiration is elevated in AOX-expressing larvae (Figure 5B). This in turn is reflected in the calculations for respiratory control ratio (RCR), which is a parameter that indicates how coupled mitochondria are by dividing the values for OXPHOS respiration by those of Leak respiration46,47. The lower RCR in AOX-expressing larvae suggests mitochondria are less coupled in these flies (Figure 5C). We speculate that the energy that is not being used for ATP synthesis is in fact being dissipated as heat, which gives AOX-expressing larvae more mobility at such an extremely stressful temperature.
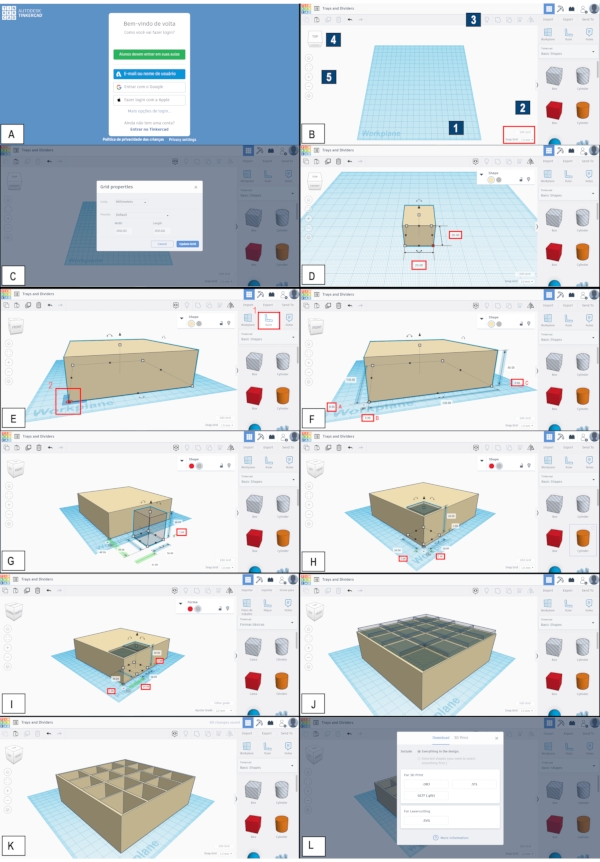
Figure 1. Modeling of a small fly vial rack using Tinkercad. (A) Access to the modeling platform is given online38using the user´s preferred password-protected credentials. (B) General view of the platform. Blue square 1 indicates the workplane, which represents the printer table, where objects are designed. Blue square 2, Basic Shapes menu, where several pre-made shapes are found. Blue square 3, a menu with useful functions, such as mirror, copy, duplicate and combine. Blue square 4, workplane movement menu, which controls rotations of the workplane to better view the object being modeled. Positioning the side of the cube using the mouse´s left button to "Top", "Bottom", "Front", "Back", "Right" and "Left" shows views of the object from different angles. Blue square 5, left menu containing other useful functions, such as zoom in and zoom out, among others. Red square, "Edit Grid" and "Snap Grip" options. (C) The Edit Grid window, where the workplane can be edited. (D) The design of a solid box (future vial rack). Red squares indicate the textboxes in which the dimensions of the box can be typed. (E) The design of a solid box (future vial rack) of 130 (length) x 130 (width) x 40 (height) mm. Red square 1 indicates the tool "Ruler"; red square 2, the lower left vertex of the box, which will become the initial point (x = 0, y = 0, z = 0) of a tridimensional Cartesian coordinate system. (F) The same solid box as in E, with red squares A-C illustrating that the lower left vertex of the box is now 0.00 mm away in all axes from the initial point of a tridimensional Cartesian coordinate system. (G) An empty (hole) box (future space for a fly vial) of 30 x 30 x 40 mm is inserted in the workplane. The red square indicates the textbox in which 2.00 should be typed to elevate the empty box 2 mm from the workplane. (H) The same empty box as in G is positioned inside the initial solid box of 130 x 130 x 40 mm by typing 2.00 in the textboxes indicated with red squares. (I) A second empty box inserted into the design illustrates how the spaces for fly vials are created one by one, based on the first empty box. The red squares indicate the textboxes that must be filled for the proper positioning of the empty boxes. (J) The design is now filled with 16 empty boxes, evenly spaced inside the initial solid box. (K) Final 3D model of the vial rack, which is now a single piece, created by grouping the initial solid box with the 16 empty boxes evenly spaced inside the solid box. See Protocol 1 for details on the whole procedure, including how to save the final model as an STL file (L). Please click here to view a larger version of this figure.
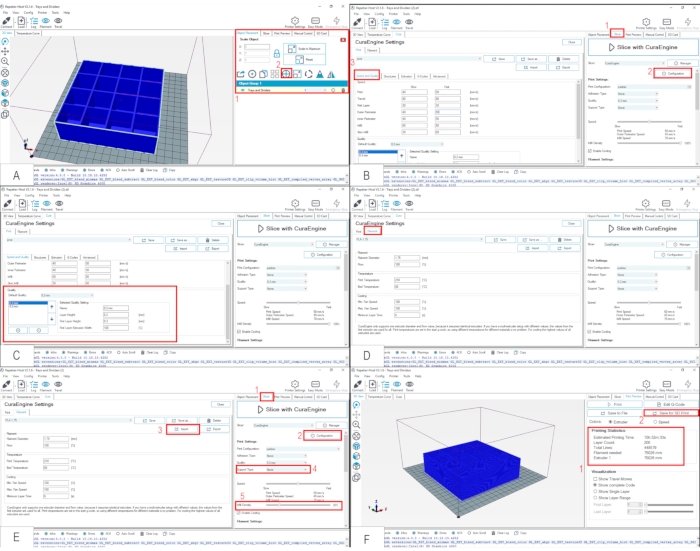
Figure 2. Slicing of the 3D vial rack model and configuring the printer using Repetier-Host. (A) The STL file containing the model of the vial rack (see Protocol 1 and Figure 1 for details) is opened with the Repetier-Host software installed on the computer. The editing menu on the right is indicated by red square 1, and the Center Object button by red square 2. (B) Initial steps to run the slicing software (see Protocol 2 for details). Red square 1 indicates the Slicer tab on the editing menu; red square 2, the Configuration button; red square 3, the Speed and Quality window, where important printer parameters such as velocity, layer thickness and holders can be defined (see the Discussion for details). (C) Detail of the Speed and Quality window. The red square indicates where the thickness of the printing layers (Layer Height and First Layer Height) can be adjusted. (D) In the Filament tab (red square), one is able to check parameters related to the filament to be used, such as the Diameter (which for most filaments is set to 1.75 mm), the Print and Bed Temperatures (respectively, the temperature to melt the filament at the printer extruder, and the temperature of the printer table which helps with adherence), and the Cooling Speed (which in general guarantees that the extruder does not overheat). (E) Final steps to run the slicing software (see Protocol 2 for details). Red squares 1 and 2 same as in (B); red square 3, the Import button, where most of printer´s adjusting parameters can be uploaded from Supplemental File 1; red square 4, Support Type option, which in this particular case of the fly vial rack should be "None"; and red square 5, Infill Density option, which should be 20% for printing most objects. The use of a support, such as for the printing of the T-Maze (see Protocol 3 for details), is necessary when the layers are not to be printed directly onto the printer table or on top of another layer. The support is particularly important to prevent collapse of structural parts that are in an arc format or of closed structures that are hollow inside, for example. (F) Visualization of the printing statistics (red square 1) under the Print Preview tab, which is calculated after the Slice with CuraEngine button (shown in E) is pressed. After pressing the Save for SD Print button (red square 2), the G-Code file should be saved in an SD card to be transported to the printer. Please click here to view a larger version of this figure.
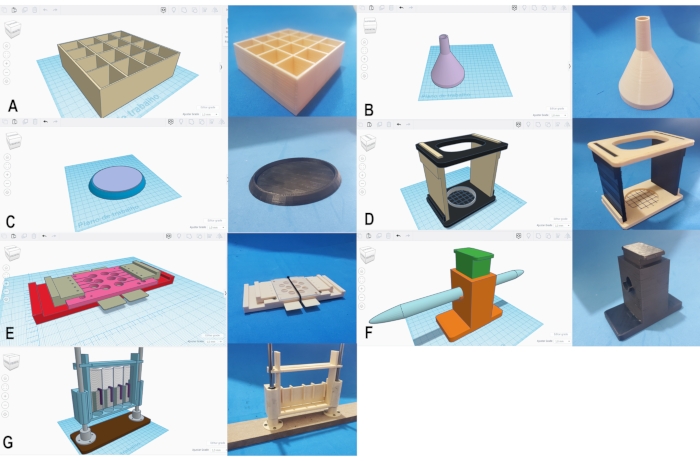
Figure 3. "Homemade" apparatuses for general fly maintenance and behavioral analyses. Side-by-side images of our model designs (left) and of the actual printed apparatuses (right) are shown: small fly rack (A), funnel (B), tapping mat support (C), Stalker (D), Fly Motel (E), T-Maze (F), and RING apparatus (G). Details on how to print and assemble these apparatuses are shown in Protocols 1-3. Note that in F, the picture of the T-Maze does not contain the two 15 mL conical tubes which would give it its "T" shape41. Please click here to view a larger version of this figure.

Figure 4. Higher mobility of AOX-expressing larvae cultured at 12 °C. Measurements of distance crawled (A) and number of body wall contractions (B) per minute by individual larva were obtained as described in Protocol 4. The fly lines used were w1118 (control, AOX-nonexpressor) and 3xtubAOX (AOX-expressor)25. Datapoints indicate means ± standard deviation of 8 biological replicates with 15 technical repetitions each. * indicates significant differences (p<0.01), according to a Student's t-test. Please click here to view a larger version of this figure.
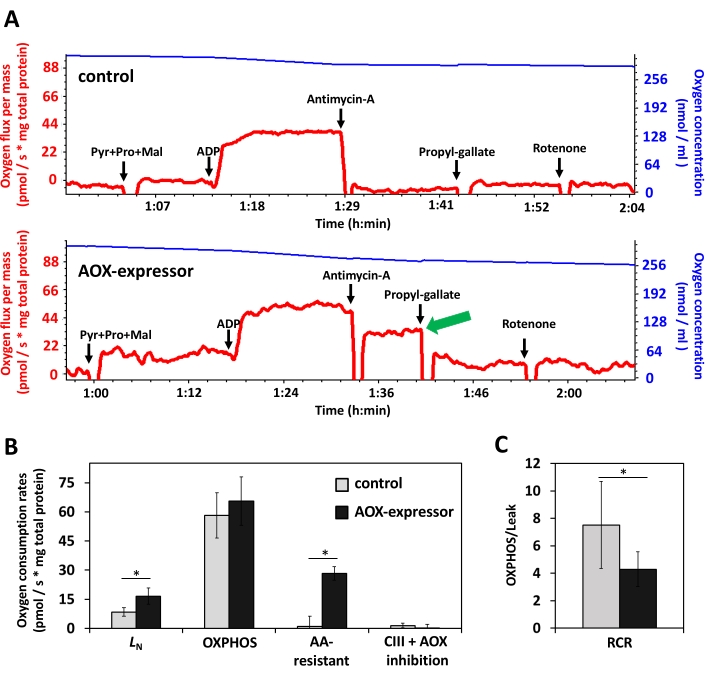
Figure 5. Higher levels of mitochondrial oxygen consumption in AOX-expressing larvae cultured at 12°C. (A) Traces of a representative experiment showing changes in real time oxygen consumption (red line) and oxygen concentration (blue line) in the chambers as the indicated substrates and inhibitors were added (black arrows) to the assay medium containing whole larva homogenates of the w1118 (control, AOX-nonexpressor) and 3xtubAOX (AOX-expressor). Pyr+Pro+Mal indicates addition of pyruvate, proline and malate, which are oxidized inside mitochondria providing NADH, substrate for complex I. The following increase in oxygen consumption is due to Leak respiration without adenylates (LN). Addition of ADP allows the ATP-synthase to release the proton gradient so that the Oxidative Phosphorylative respiratory state (OXPHOS) is achieved. Addition of antimycin A inhibits complex III (CIII), completely abolishing oxygen consumption in the AOX-nonexpressing control sample (top graph), and allowing the antimycin A-resistant respiration activity of AOX (green arrow) to be measured in the AOX-expressing sample (bottom graph). Addition of propyl-gallate, an AOX inhibitor, followed by the complex I inhibitor rotenone, certifies that mitochondrial respiration is totally abolished in both lines, allowing baseline oxygen consumption to be established. The first 50-60 min of the experiments, prior to the addition of Pyr+Pro+Mal, show the traces during stabilization of the oxygraph system, and were deliberately omitted here. The traces before addition of the sample homogenates are used for calculation of air oxygen saturation (see Protocols 5 and 6 for details). (B) Quantification of the oxygen consumption data shown in A, averaged with that of 3-5 other biological replicates (± standard deviation). AA-resistant, antimycin A-resistant respiration; CIII + AOX inhibition, residual respiration. (C) Respiratory control ratio (RCR) was calculated as the average ratio between OXPHOS and LN respirations of the data shown in B and that of 3-5 other biological replicates (+/- standard deviation), to estimate mitochondrial OXPHOS-coupling efficiency. We have previously measured Leak respiration in the presence of oligomycin (LOmy) and determined no differences between LOmy and LN (data not shown). Using LN to compute RCR allows us to measure the antimycin A-resistant respiration by AOX in the same experiment. * indicates statistical differences (p<0.05), according to Student's t-tests. Please click here to view a larger version of this figure.
Supplemental File 1. Please click here to download this File.
Supplemental File 2. Please click here to download this File.
Supplemental File 3. Please click here to download this File.
Supplemental File 4. Please click here to download this File.
Supplemental File 5. Please click here to download this File.
Supplemental File 6. Please click here to download this File.
Supplemental File 7. Please click here to download this File.
Supplemental File 8. Please click here to download this File.
Supplemental File 9. Please click here to download this File.
Supplemental File 10. Please click here to download this File.
Supplemental File 11. Please click here to download this File.
Discussion
The 3D-printing protocols and STL files provided here are intended to facilitate the setup of a new flylab or to increase the repertoire of apparatuses in an existing Drosophila behavioral facility, using "homemade" equipment. The 3D-printing strategy may be particularly useful in developing countries such as Brazil, where research using Drosophila as a model organism for studying human biology appears to be underrepresented, and specialized equipment is costly. Our protocols provide instructions on how to create the plastic framework and to assemble relatively simple devices, such as vial racks and apparatuses for memory, courtship and climbing assays, which may only need extra simple non-printable pieces for complete functionality. For proper printing results, the user may need to adjust a few printing parameters based on his/her own printer. In Figure 2B, under "Speed and Quality" (red square 3), one can find important settings that may determine the quality of the final printed piece. "Print" controls the range of speed at which the printer extruder works to form the 3D piece; the lower the speed, the higher the final quality and the total time needed to complete the job. In "First Layer", one should consider the printing speed of the layer that will be placed directly onto the printer table. It is important that this is slow (30 mm/s), as any imperfections in the first layer may alter all of the layers above, causing deformities in the final piece. For the same reasons, we choose higher thickness for the first layer ("First Layer Height: 0.3 mm"), whereas the remaining layers can be thinner ("Layer Height", red square in Figure 2C) without detriments to the final piece.
As the interested Drosophilist becomes more familiarized with the 3D-priting methods by creating the devices presented here, we recommend him/her to invest in more complex equipment that can provide more streamlined quantitative data for different behavioral parameters. One such equipment includes the FlyPi15, which is a relatively affordable, open-source platform for measuring behaviors of small model animals, including Drosophila, that can be controlled by optogenetic and/or thermogenetic tools, due to the attached LED-based fluorescence microscope and the Peltier-based temperature stimulator modules. Another example of a more sophisticated equipment that can be home-made is the ethoscope48, a platform for high-throughput, real-time tracking and profiling of small animal behavior. For these, nevertheless, one must also invest in electronic components, such as cables, circuits, cameras, lens, among other parts, and follow the instructions provided by the original designers to assemble the parts into the 3D-printed framework and connect with the software49,50.
Among all model designs we present here, those for the tapping mat support and the funnel appear to be basically dispensable for a Drosophilist in a developed country. However, it is important to emphasize that basic supplies for a flylab in Brazil are not easily purchasable. For example, we are not aware of any company that produces polypropylene fly vials and bottles in the country; these usually serve a single-use purpose in flylabs. They must then be imported and, as a result, they are relatively expensive and rarely ready for delivery when available in the national market. The large number of local companies that do glasswork, however, can easily provide us with customized reusable glass vials and bottles (such as those used by Thomas Hunt Morgan in the original Fly Room), which must be carefully cushioned when tapped to avoid breaking. Tapping mats and their supports are therefore extremely useful. We have not been able to 3D-print fly vials and bottles efficiently, as the printed pieces are not transparent enough for proper observation of the flies inside, even when using transparent filament (data not shown).
Recently, the company Polymaker developed the Polysmooth, a filament designed specifically for post-processing and removal of the printing layer lines, allowing the printed devices to have a high degree of transparency. This may be extremely useful for printing fly vials in the future. Regarding funnels, although inexpensive and easily found in any supermarket or hardware store, they often have long tips and round cups, which are impractical for use in the lab. In this case, 3D-printing provided us with funnels customized for our needs.
Device customization is perhaps the most important aspect of the 3D-printing strategy, which can be employed by flylab members in developed and developing countries alike. We in fact have also customized the Fly Motel, the T-Maze and the RING apparatus, whose original designs were published elsewhere40,41,42. The original Fly Motel design, for example, has more arenas and is therefore significantly larger in size40. Printing such large apparatus would not have been feasible using our RepRap printer because of the limited space of its printing table (20 cm x 20 cm). In addition, recordings encompassing all individual arenas of the original Fly Motel design simultaneously would have generated poorer quality videos using our cameras, which could compromise software tracking of the individual animals and, ultimately, the behavioral analysis of the pair of flies in each individual arena. Moreover, the Fly Motel and the framework of the T-Maze were originally designed to be made of acrylic, which is significantly more expensive and allows less flexibility when assembling modular apparatuses; in other words, the actual acrylic pieces must have the exact size shown in the design, so that they can fit, assemble and form a single device40. Instead, the PLA-printed pieces of the Fly Motel and the T-Maze are rigid, yet show some flexibility during assembly, in addition to having no known toxicity. 3D-printing the T-Maze using PLA also has another advantage over acrylic, as the former does not allow ambient light to penetrate the chamber inside the apparatus, allowing the flies to properly choose either of the two lateral conical tubes where the light should in fact play its essential role in these behavioral assays41.
Although simple, the Stalker is our own creation, designed to be very versatile. In addition to using it with any camera available as a stand-alone device so videos and pictures of flies can be made and analyzed on independent software, such as ImageJ51, it may also serve the purpose of a removable appendment to our own version of the ethoscope, by inserting it into the latter´s internal recordable space. The STL files we make available with this article can be opened with Tinkercad to be even further altered with relative ease, before the interested researcher decides to 3D-print any of the devices, including our original Stalker. We make ourselves available by email to clarify any particular steps of our protocols, and to help with new designs of interest. We also encourage the reader to find important information on websites of "makers culture" communities dedicated to design and produce lab equipment that is affordable for biologists in general: the Open Neuroscience52 and the 3D Printable Science53.
We recognize that even with all the designed apparatuses presented here, some behavioral assays still need to be performed "manually". Although this may introduce artifacts and subjectivity, it allowed us to analyze parameters that cannot be easily quantified using open-source software we have in our lab, such as the peristaltic movements of the larva body wall. Automated measurements of these movements can, however, be made using the FlyPi platform15. To test how the introduction of the non-proton pumping terminal oxidase AOX into the fly´s OXPHOS system may influence behavior, we made use of manual counting of larval peristaltic movements under the microscope. Because we used the extreme temperature of 12 °C and larval mobility is usually very limited under these conditions54,55, manually measuring the distance travelled by the larvae along with counting body wall contractions turned out to be entirely feasible. We have shown previously that larval development is faster and larval viability is higher when AOX is expressed in flies cultured under stressful low temperatures, with more pronounced results at 12 °C34. The hypothesis to explain these was based on the known thermogenic properties of plant AOX56,57,58: the enzyme activity uncouples mitochondria, generating heat and consequently accelerating larval metabolism. Showing here that AOX-expressing larvae are significantly more mobile at 12 °C (Figure 4) is consistent with such a hypothesis.
Also consistent with the thermogenesis hypothesis is our mitochondrial respirometry data (Figure 5). We first developed the mitochondrial oxygen consumption protocol described here to be used with larval homogenates in an Oroboros O2k oxygraph at 12 °C. Condensation on the outside of the chambers due to the colder temperature inside was an initial concern for the long-term function of the equipment, but this does not appear to have caused any significant changes in the oxygraph performance throughout several years of use (data not shown). The use of total larval homogenates provides us with an estimate of mitochondrial performance in general, which is an important step when ubiquitously expressing AOX. Unlike respirometry experiments using whole adult homogenates, in which the flight muscle mitochondria are the most abundant mitochondria in the samples59,60 the tissue(s) that most contribute(s) to mitochondrial respiration in larval homogenates has(ve) not yet been determined experimentally. Our protocol may certainly be advantageous in the pursuit of this goal, and in the investigation of the possible AOX-driven thermogenesis in a tissue-specific fashion. Using whole larva homogenates, however, requires significantly more cleaning and maintenance of the O2k chambers and oxygen sensors for proper continuing function of the equipment. We recognize that the high abundance of lipids and proteins stored in larvae is an important issue, as these with time may deposit on the interior walls of the assay chambers. Frequently, we have to clean the chambers with concentrated hydrochloric acid for completely removal of these deposits, following instructions from the manufacturer´s website43. This process is time-consuming as the chambers must be removed from the equipment for a whole day of cleaning, and the equipment must be recalibrated when reassembled. This is, nevertheless, extremely important to prevent accumulation of lipid-soluble mitochondrial inhibitors that may interfere with the oxygen consumption assays.
Here, we measured three mitochondrial respiratory conditions in the presence of substrates whose oxidation provides electrons to CI, which in turn are finally used for O2 reduction in the cytochrome c segment of the respiratory chain (CIII and CIV) or by AOX. LN is sustained by electron transfer through these complexes and AOX, as a result of proton leakage from the mitochondrial intermembrane space to the matrix (intrinsic uncoupling). The OXPHOS condition is sustained basically by the same electron transfer reactions, which is in this case stimulated by the action of the ATP synthase, as it permits the dissipation of the proton gradient and the condensation of ADP and inorganic phosphate (coupled respiration)61. The antimycin A-resistant respiration, however, is similar to OXPHOS, but driven solely by AOX as the terminal oxidase. AOX-expressing larvae present strong antimycin A-resistant respiration (Figure 5A and B), which allows the development of these flies in food containing toxic levels of antimycin A24. We also observed significantly higher LN in the presence of AOX (Figure 5A and B), which results in a lower RCR (Figure 5C). This estimate of lower mitochondrial coupling indicates higher energy dissipation, which is again consistent with a thermogenic role for AOX in Drosophila larvae cultured at low temperatures. However, additional experiments are warranted to show how much heat AOX produces and how this changes metazoan mitochondrial metabolism. This may include investigations testing other mitochondrial electron transfer pathways and other temperatures. Notably, the Oroboros high resolution respirometry system is a versatile equipment, allowing different combination of protocols to test the mitochondrial electron transfer system in different ways. In addition, and perhaps more importantly, the protocols can be optimized as needed and the interested Drosophilist can count on a large online community of O2k users specialized in mitochondria of diverse tissues, and on the open-source materials provided by the company in their website43.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Emily A. McKinney for English editing of the manuscript. G.S.G. was supported by a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number 141001/2019-4). M.T.O. would like to acknowledge funding from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant numbers 2014/02253-6 and 2017/04372-0), and the CNPq (grant numbers 424562/2018-9 and 306974/2017-7). C.A.C.-L. would like to acknowledge internal financial support from the Universidade do Oeste Paulista. The work with genetically modified Drosophila lines was authorized by the Local Biosafety Committee (CIBio) of the Faculdade de Ciências Agrárias e Veterinárias de Jaboticabal, under the protocols 001/2014 and 006/2014, and by the National Technical Committee on Biosafety (CTNBio), under the protocols 36343/2017/SEI-MCTIC, 01200.706019/2016-45, and 5488/2017.
Materials
| 3D Printer RapRep | A popular 3D-printer based on the Prusa I3 DIY mode, instructions available in https://www.instructables.com/Building-a-Prusa-I3-3D-Printer-Revisited/ | ||
| 3xtubAOX fly line | Howy Jacobs´s lab, Tampere University | Drosophila line expressing the AOX gene from C. intestinalis under the control of the constitutive α-tubulin promoter. 5 and 6 copies of this construct are present in males and females in homo/hemizigosity, respectively, one in each of the chromosomes X, 2 and 3. | |
| Acrylic plate | 60 x 60 x 3 mm | ||
| ADP | Sigma-Aldrich | A2754 | Adenosine 5′-diphosphate sodium sal (CAS number 20398-34-9); ≥95%; molecular weight = 427.20 g/mol; solubility in water at 50 mg/ml |
| Antimycin-A | Sigma-Aldrich | A8674 | Antimycin A from Streptomyces sp. (CAS number 1397-94-0); molecular weight ~ 548.63 g/mol; solubility in 95% ethanol at 50 mg/mL |
| Agar | Kasv | K25-611001 | For bacteriologal use; powder; solidifying agent (12-20 g/L) |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | A7030 | Heat shock fraction, protease free, fatty acid free, essentially globulin free (CAS number 9048-46-8);pH 7; ≥98%; solubility in water 40g/ml |
| Deionized water | |||
| EGTA | Sigma-Aldrich | E4378 | Ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (CAS number 67-42-5); ≥97%; molecular weight = 380.35g/mol |
| Ethanol 99.5% | |||
| Ethylene-vinyl acetate foam | Can be replaced with thick pieces of cotton | ||
| Graph paper | 0.2 cm2 grid | ||
| Hepes | Sigma-Aldrich | H4034 | 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (CAS number 7365-45-9), BioPerformance Certified; ≥99,5% (titration), cell cultured tested; molecular weight =238.30g/mol |
| Homogenizer | Sartorius | Hand glass homogenizer (S), 1 mL; composed of a cylinder made of borosilicate glass plus plunger S; often used for simple sample preparation, e.g. crushing of tissue samples. | |
| KCl | Amresco | 0395-2 | Potassium chloride (CAS number 7447-40-7); ≥99,0%; molecular weight = 74.55g/mol |
| KH2PO4 | Sigma-Aldrich | P5379 | Potassium phophate monobasic (CAS number 7778-77-0); ReagentPlus; molecular weight = 136.09g/mol |
| Linear bearings (LM8UU) | 8 mm, any brand | ||
| Malate | Sigma-Aldrich | M1000 | L-(-)-Malic acid (CAS number 97-67-6); ≥95-100%; molecular weight = 134.09 g/mol), solubility in water: 100 mg/mL. A solution is pH adjusted to approximately 7.0. |
| MgCl2 | Amresco | 0288-1KG | Magnesium chloride, hexahydrate (CAS number 7791-18-6); 99%-102%; molecular weight = 203.3g/mol |
| Microcentrifuge tubes | 1.5mL; Graduated every 100µL, autoclavable | ||
| Na2HPO4 | Amresco | 0348-1KG | Sodium phosphate, dibasic, heptahydrate (CAS number 7782-85-6); 98-102%; molecular weight = 268.07 g/mol |
| NaCl | Honeywell | 31434-1KG | Sodium chloride (CAS number 7647-14-5); ≥99,5%; molecular weight 58,44g/mol. For laboratory use only. |
| Oxigraph-O2k | Oroboros | 10000-02 | Series D-G; O2k-Core: includes O2k-Main Unit with stainless steel housing, O2k-Assembly Kit, two OroboPOS (polarographic oxygen sensors) and OroboPOS-Service Kit, DatLab software, the ISS-Integrated Suction System and the O2k-Titration Set. |
| Permanent marker | Preferably black | ||
| Petri dishes | 90 X 15 mm dishes; commonly used for bacteriological culture | ||
| PLA 3D Printing Filament | Quantum3D Printing | http://quantum3dprinting.com/ | High quality polylatic acid filament (PLA), strongly recomended, (1.0 kg Roll), any brand |
| Proline | Sigma-Aldrich | P0380 | L-Proline (CAS number 147-85-3); powder; 99%; molecular weight = 115.13 g/mol |
| Propyl gallate | Sigma-Aldrich | P3130 | Propyl gallate (CAS number 121-79-9); powder; ≥98%; molecular weight = 212.2 0g/mol; solubility in ethanol at 50 mg/ml |
| Pyruvate | Sigma-Aldrich | P2256 | Sodium pyruvate (CAS number 113-24-6), ≥99%; molecular weight = 110.04 g/mol; solubility in water at 100 mg/mL |
| Rectified shafts | 8 x 300 mm, any brand | ||
| Rotenone | Sigma-Aldrich | R8875 | Rotetone (CAS number 83-79-4); ≥95%, molecular weight 394.42 g/mol |
| Rubber bands | Can be replaced with pieces of a string | ||
| Screwdriver | To assemble some of the 3D-printed apparatuses | ||
| Screews | M3 x 8 mm | ||
| SD Card | At least 32Mb in size; usually provided with 3D printers | ||
| Software Repetier Host | Hot-World GmbH & Co. KG | https://www.repetier.com/ | Excellent slicing software, available free of cost |
| Software Tinkercad | Autodesk | https://www.tinkercad.com | 3D model design software, available free of cost |
| Stereomicroscope | Leica | M-80 | Stereomicroscope, zoom 7.5-60X + Leica cls 150 led light source |
| Sucrose | Merck | 107,651,000 | Sucrose for microbiology use (CAS number 57-50-1); |
| Tris | Amersham Biosciences | 17-1321-01 | Tris (hydroxymethyl)-aminomethane (CAS number 77-86-1); 99,8-100.1%; molecular weight 121.14 g/mol |
| Tweezer/forceps | Stark | ST08710 | Histological tweezer, straight, round tip, 12 cm, AISI-410 stainless steel |
| w1118 fly line | Howy Jacobs´s lab, Tampere University | Drosophila line used as genetic background control for 3XtubAOX | |
| Wood plate | 240 x 60 x 20 mm | ||
| Zip tights | 2 x 210 mm, any brand |
References
- . Drosophila Model Filter Available from: https://pubmed.ncbi.nlm.nih.gov/?term=%22drosophilia+model%22&filter=datesearch.y_5 (2021)
- . A Look at Trends in NIHS Model Organism Research Support Available from: https://nexus.od.nih.gov/all/2016/07/14/a-look-at-trends-in-nihs-model-organism-research-support/> (2021)
- . Drosophila Available from: https://bv.fapesp.br/pt/metapesquisa/?q=drosophila (2021)
- . Metapesquisa Available from: https://bv.fapesp.br/pt/metapesquisa/ (2021)
- Jennings, B. H. Drosophila-a versatile model in biology & medicine. Materials Today. 14 (5), 190-195 (2011).
- Brandt, A., Vilcinskas, A. The Fruit Fly Drosophila melanogaster as a Model for Aging Research. Yellow Biotechnology I. Advances in Biochemical Engineering/Biotechnology. 135, 63-77 (2013).
- Yang, D. Simple homemade tools to handle fruit flies-drosophila melanogaster. Journal of Visualized Experiments. (149), e59613 (2019).
- Cheluvappa, R., Scowen, P., Eri, R. Ethics of animal research in human disease remediation, its institutional teaching; and alternatives to animal experimentation. Pharmacology Research and Perspectives. 5 (4), (2017).
- . Plan Alto.gov Available from: https://www.planalto.gov.br/ccivil_03/_ato2004-2006/2005/decreto/d5591.htm (2021)
- . Revistapesquisa.fapesp.br Available from: https://revistapesquisa.fapesp.br/en/supply-side-research-constraints/ (2021)
- Nascimento, S., Pólvora, A. Maker Cultures and the Prospects for Technological Action. Science and Engineering Ethics. 24 (3), 927-946 (2018).
- Maia Chagas, A., Prieto-Godino, L. L., Arrenberg, A. B., Baden, T. The €100 lab: A 3D-printable open-source platform for fluorescence microscopy, optogenetics, and accurate temperature control during behaviour of zebrafish, Drosophila, and Caenorhabditis elegans. PLoS Biology. 15 (7), (2017).
- Baden, T., Chagas, A. M., Gage, G., Marzullo, T., Prieto-Godino, L. L., Euler, T. Open Labware: 3-D Printing Your Own Lab Equipment. PLOS Biology. 13 (3), 1002086 (2015).
- Zhou, B., Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. Journal of Clinical Investigation. 128 (9), 3716-3726 (2018).
- Hock, D. H., Robinson, D. R. L., Stroud, D. A. Blackout in the powerhouse: Clinical phenotypes associated with defects in the assembly of OXPHOS complexes and the mitoribosome. Biochemical Journal. 477 (21), 4085-4132 (2020).
- Juszczuk, I. M., Rychter, A. M. Alternative oxidase in higher plants. Acta Biochimica Polonica. 50 (4), 1257-1271 (2003).
- McDonald, A. E. Alternative oxidase: An inter-kingdom perspective on the function and regulation of this broadly distributed “cyanide-resistant” terminal oxidase. Functional Plant Biology. 35 (7), 535-552 (2008).
- McDonald, A. E., Vanlerberghe, G. C., Staples, J. F. Alternative oxidase in animals: Unique characteristics and taxonomic distribution. Journal of Experimental Biology. 212, 2627-2634 (2009).
- McDonald, A., Vanlerberghe, G. Branched Mitochondrial Electron Transport in the Animalia: Presence of Alternative Oxidase in Several Animal Phyla. IUBMB Life (International Union of Biochemistry and Molecular Biology: Life. 56 (6), 333-341 (2004).
- McDonald, A. E., Costa, J. H., Nobre, T., De Melo, D. F., Arnholdt-Schmitt, B. Evolution of AOX genes across kingdoms and the challenge of classification. Alternative Respiratory Pathways in Higher Plants. , 267-272 (2015).
- Fernandez-Ayala, D. J. M., et al. Expression of the Ciona intestinalis Alternative Oxidase (AOX) in Drosophila Complements Defects in Mitochondrial Oxidative Phosphorylation. Cell Metabolism. 9 (5), 449-460 (2009).
- Kemppainen, K. K., et al. Expression of alternative oxidase in Drosophila ameliorates diverse phenotypes due to cytochrome oxidase deficiency. Human Molecular Genetics. 23 (8), 2078-2093 (2014).
- Andjeiković, A., Kemppainen, K. K., Jacobs, H. T. Ligand-bound geneswitch causes developmental aberrations in drosophila that are alleviated by the alternative oxidase. G3: Genes, Genomes, Genetics. 6 (9), 2839-2846 (2016).
- Hakkaart, G. A. J., Dassa, E. P. E. P., Jacobs, H. T., Rustin, P. Allotopic expression of a mitochondrial alternative oxidase confers cyanide resistance to human cell respiration. EMBO Reports. 7 (3), 341-345 (2006).
- Dassa, E. P., et al. Expression of the alternative oxidase complements cytochrome c oxidase deficiency in human cells. EMBO Molecular Medicine. 1 (1), 30-36 (2009).
- Szibor, M., et al. Broad AOX expression in a genetically tractable mouse model does not disturb normal physiology. DMM Disease Models and Mechanisms. 10 (2), 163-171 (2017).
- El-Khoury, R., Kaulio, E., Lassila, K. A., Crowther, D. C., Jacobs, H. T., Rustin, P. Expression of the alternative oxidase mitigates beta-amyloid production and toxicity in model systems. Free Radical Biology and Medicine. 96, 57-66 (2016).
- Mills, E. L., et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 167 (2), 457-470 (2016).
- Giordano, L., et al. Alternative Oxidase Attenuates Cigarette Smoke-induced Lung Dysfunction and Tissue Damage. American Journal of Respiratory Cell and Molecular Biology. 60 (5), 515-522 (2019).
- Camargo, A. F., et al. Xenotopic expression of alternative electron transport enzymes in animal mitochondria and their impact in health and disease. Cell biology International. 42 (6), 664-669 (2018).
- Saari, S., et al. Alternative respiratory chain enzymes: Therapeutic potential and possible pitfalls. Biochimica et Biophysica Acta – Molecular Basis of Disease. 1865 (4), 854-866 (2019).
- . Instructables.com Available from: https://www.instructables.com/Building-a-Prusa-I3-3D-Printer-Revisted/ (2021)
- . Tindercad.com Available from: https://www.tinkercad.com/ (2021)
- . Repetier.com Available from: https://www.repetier.com/ (2021)
- . Tinkercad.com Available from: https://www.tinkercad.com/login (2021)
- . Knowledge.autodesk.com Available from: https://knowledge.autodesk.com/support/revit-products/learn_explore/caas/CloudHelp/cloudhelp/2016/ENU/Revit-Model/files/GUID-B89AD692-C705-458F-A638-EE7DD83D694C-htm.html (2021)
- Koemans, T. S., et al. Drosophila courtship conditioning as a measure of learning and memory. Journal of Visualized Experiments. (124), e55808 (2017).
- Ali, Y. O., Escala, W., Ruan, K., Zhai, R. G. Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. Journal of Visualized Experiments. (49), (2011).
- Nichols, C. D., Becnel, J., Pandey, U. B. Methods to assay Drosophila behavior. Journal of visualized experiments JoVE. (61), e3795 (2012).
- . Oroboros Available from: https://www.tinkercad.com/login (2021)
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72 (1-2), 248-254 (1976).
- . Tinkercad Available from: https://www.tinkercad.com/login (2021)
- Morton, J. D., Barnes, M. F., Zyskowski, R. F. Respiratory control ratio: A computer simulation of oxidative phosphorylation. Biochemical Education. 24 (2), 110-111 (1996).
- Chance, B., Williams, G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. The Journal of Biological Chemistry. 217 (1), 383-393 (1955).
- Geissmann, Q., Garcia Rodriguez, L., Beckwith, E. J., French, A. S., Jamasb, A. R., Gilestro, G. F. Ethoscopes: An open platform for high-throughput ethomics. PLOS Biology. 15 (10), 2003026 (2017).
- . Github.com Available from: https://www.tinkercad.com/login (2021)
- . Gilestrolab.github.io Available from: https://www.tinkercad.com/login (2021)
- . Imagej.nih.gov Available from: https://www.tinkercad.com/login (2021)
- . Open-Neuroscience.com Available from: https://www.tinkercad.com/login (2021)
- . Appropedia.org Available from: https://www.tinkercad.com/login (2021)
- McParland, A. L., Follansbee, T. L., Ganter, G. K. Measurement of larval activity in the Drosophila activity monitor. Journal of Visualized Experiments. , (2015).
- Schou, M. F., Kristensen, T. N., Pedersen, A., Karlsson, B., Loeschcke, V., Malmendal, A. Metabolic and functional characterization of effects of developmental temperature in Drosophila melanogaster. American Journal of Physiology – Regulatory Integrative and Comparative Physiology. 312 (2), 211-222 (2017).
- Meeuse, B. J. D. Thermogenic Respiration in Aroids. Annual Review of Plant Physiology. , (1975).
- Watling, J. R., Robinson, S. A., Seymour, R. S. Contribution of the alternative pathway to respiration during thermogenesis in flowers of the sacred lotus. Plant Physiology. , (2006).
- Inaba, Y. I., et al. Alternative oxidase capacity of mitochondria in microsporophylls may function in cycad thermogenesis. Plant Physiology. 180 (2), 743-756 (2019).
- Sanz, A., Stefanatos, R., McIlroy, G. Production of reactive oxygen species by the mitochondrial electron transport chain in Drosophila melanogaster. Journal of Bioenergetics and Biomembranes. 42 (2), 135-142 (2010).
- Miwa, S., St-Pierre, J., Partridge, L., Brand, M. D. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radical Biology and Medicine. 35 (8), 938-948 (2003).
- Gnaiger, E. Mitochondrial Pathways and Respiratory Control An Introduction to OXPHOS Analysis. Bioenergetics Communications. 2, (2020).

.
