Quantitation of Rabies Virus in Various Bovine Brain Structures
Summary
This protocol presents a qRT-PCR-based approach for determining the rabies virus nucleoprotein (N) gene copy number within various bovine brain anatomical structures using in vitro transcription.
Abstract
Bovine paralytic rabies (BPR) is a form of viral encephalitis that is of substantial economic importance throughout Latin America, where it poses a major zoonotic risk. Here, our objective was to utilize a laboratory protocol to determine the relative copy number of the rabies virus (RABV) genome in different bovine brain anatomical structures using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). qRT-PCR quantifies the specific number of gene copies present in a sample based on fluorescence emitted after amplification that is directly proportional to the amount of target nucleic acid present in the sample. This method is advantageous owing to its short duration, reduced risk of contamination, and potential to detect viral nucleic acids in different samples more easily compared to other techniques. The brains of six rabid animals were divided into six anatomical structures, namely the Ammon’s horn, cerebellum, cortex, medulla, pons, and thalamus. All brains were identified as positive for RABV antigens based on a direct immunofluorescence test. The same anatomical structures from the brains of four RABV-negative bovines were also assessed. RNA was extracted from each structure and used for qRT-PCR. An assay was performed to determine the copy numbers of RABV genes using an in vitro transcribed nucleoprotein gene. The standard curve used to quantify viral RNA exhibited an efficiency of 100% and linearity of 0.99. Analysis revealed that the cortex, medulla, and thalamus were the ideal CNS portions for use in RABV detection, based on the observation that these structures possessed the highest levels of RABV. The test specificity was 100%. All samples were positive, no false positives were detected. This method can be used to detect RABV in samples that contain low levels of RABV during diagnosis of BPR.
Introduction
Rabies can be confirmed ante-mortem and post-mortem by various techniques that enable the detection of viral nucleic acids in the brain, skin, urine, or saliva1. Detection of the rabies virus nucleoprotein (N) gene is primarily used for rabies diagnosis by molecular tests. This gene is also used for viral genotyping. Rabies can be diagnosed in animals using any portion of the affected brain; however, to exclude the possibility of rabies, tissue from at least two regions in the brain must be tested2. Several diagnostic methods exist for rabies detection in animals; however, the direct immunofluorescence test remains the standard reference technique3. Other tests include biological tests that incorporate mouse inoculation, infection in tissue culture, and polymerase chain reaction (PCR)4. All these techniques are recommended by the World Health Organization (WHO) and World Organization of Animal Health (OIE) for the diagnosis of rabies in humans and animals, respectively5.
Nucleic acid detection and amplification techniques have revolutionized the diagnosis of rabies in recent years6 and these techniques play an important role in the ante mortem diagnosis of human rabies. Several PCR-based tests have been evaluated to complement conventional tests for ante-mortem and post-mortem rabies diagnoses7,8,9,10. Most assays target rabies viral nucleoprotein gene for amplification which is the most highly conserved region in the viral genome1,11. In the last 20 years, various molecular assays have been developed to diagnose RABV, and some of these assays have been used for virus characterization. Most trials have aimed to detect conserved genes within the viral genome, most commonly by using conventional or quantitative real-time polymerase chain reaction (qRT-PCR) assays12,13.
PCR is a highly sensitive diagnostic technique that can detect the genome of a given pathogen within tissues, even when these tissues are decomposed. Using PCR-based approaches, minimal quantities of an infectious agent can be detected in a clinical sample through the selective and repetitive amplification of a DNA nucleotide sequence14. qRT-PCR that incorporates fluorescent probes (e.g., TaqMan) or DNA binding dyes (e.g., SYBR Green) has been used in trials to diagnose RABV both ante– and post– mortem with high sensitivity; however, such an approach requires specialized equipment. To overcome this limitation, reverse transcription loop-mediated isothermal amplification (RT-LAMP) has been suggested, based on its low cost, simplicity, and desirable characteristics for the detection of RABV. This assay is particularly important as it can be used in developing countries15.
qRT-PCR is based on the detection and quantification of a molecule, where fluorescent signal increases are in direct proportion to the amount of PCR product in a single reaction. As the number of copies of the nucleic acid target increases, so does the fluorescence. Non-specific intercalating dyes such as the SYBR Green DNA-binding dye or sequence-specific oligonucleotide probes carrying a fluorophore and a quencher are commonly used to provide the fluorescent readout in qRT-PCR. This assay offers advantages over conventional RT-PCR that include a shorter test time (2–4 h), reduced risk of contamination due to the closed tube system (lack of post-PCR manipulation of amplified products), and the ability to detect different targets simultaneously16. qRT-PCR can be used to diagnose rabies ante-mortem from saliva and other samples. This assay can also be used as a universal real-time test for the detection of different Lyssavirus species or lineages of RABV15. In this combo RT-PCR approach, two reactions are used. The first detects different genetic linages of RABV, and the second detects the Lyssavirus species. Both steps involve qRT-PCR assays, where the first uses hybridization probes and the second uses dyes15. Owing to the large number of tests that have demonstrated successful molecular detection of RABV using this technique, the current OIE Terrestrial Manual (2018) recommends the use of PCR for the molecular detection of RABV17.
Mexico is a country with considerable livestock potential. The states with the highest livestock production contain both humid tropical regions and dry regions that are at risk for rabies outbreaks due to the presence of the vampire bat Desmodus rotundus, the main transmitter of rabies. Therefore, it is essential to develop more tools for the prevention and control of bovine paralytic rabies (BPR) in México. Based on this, the aim of this study was to use quantitative qRT-PCR to determine the number of viral particles in different anatomical structures of bovine brains following death due to rabies infection.
Six brains obtained from RABV-positive bovines were donated by an external laboratory for the use in the development of qRT-PCR protocol described below. The bovine brain structure samples were cold-chain transported to the INIFAP CENID-MA laboratory BSL-2 facility and stored at -80 °C until use. Brains were obtained from animals from the states of Campeche, Yucatán, and Querétaro. Prior to the receipt, various structures were dissected from the brains. These structures included the Ammon’s horn, cerebellum, cortex, medulla, pons, and thalamus18,19. Genetic material was extracted as described below. RABV diagnosis was confirmed using direct fluorescent antibodies (DFA)20. As a positive control, mouse brains that were inoculated with RABV21 were used. Additionally, four RABV-negative cattle brains (as determined by DFA) were used as negative controls.
Protocol
This study was approved by and conducted in strict accordance with the recommendations for the use of animals provided by the Institutional Animal Care and Use Committee (IACUC) of the Centro Nacional de Investigación Disciplinaria en Microbiología Animal (CENID-MA).
1. Samples
- Use different areas of the brain dissected from 6 different rabies-positive bovine brains for RT-PCR analysis.
2. Direct fluorescent antibodies (DFAs) to confirm rabies
NOTE: Detection of the rabies antigen by DFA is a qualitative method to determine the presence of the rabies nucleoprotein using fluorescein-labeled antibodies. The test was performed using the protocol provided by Dean et al. (1966)20, as described below.
- Make two impressions from each tissue dissected from different brain structures on a sterile slide of approximately 15 mm diameter. Use a wooden applicator to smear an approximately 3 mm3 portion of the tissue onto the sterile slide. To create the smear, gently press the wooden applicator against the slide manually.
- Fix the sample smear slides by submersing the slides in 100% acetone for 1 h at -20 °C. After incubation, dry the slides on a dry cloth at 21 °C for 30 min.
- Add 50 µL of the fluorescein-labeled anti-nucleoprotein antibody to each brain smear at a 1:10 dilution by dispensing through a syringe.
- Incubate the slides with the conjugate in a humid chamber at 37 °C for 1 h.
- After incubation, wash the slides twice with water and with PBS to eliminate excess conjugate. Allow the slides to dry at 21 °C. Carefully blot to remove excess liquid and then briefly air-dry prior to mounting.
- Add a drop of 50% phosphate glycerin to cover the surface of the section, and then cover with a coverslip. Observe the sections under an epifluorescence microscope at 40x magnification.
- Process and observe the positive and negative controls (positive control: mouse brains inoculated with RABV; negative controls: RABV-negative bovine brains) in the same manner as the samples.
3. Generating positive control for RT-PCR
- Obtain BHK-21 cells from the germplasm bank. Use cells up to the 70th passage to replicate the RABV Evelyn-Rokitnicki-Abelseth (ERA) strain using passage 2.
- Maintain and propagate BHK-21 cells in bottles containing minimum essential medium (MEM) supplemented with 10% fetal calf serum (FCS) at 37 °C in the presence of CO2 (5%) and under humid conditions. Grow the cells in 25 cm2 culture flasks until they reach 80% confluence and then subculture.
- Remove the culture medium from the cells and wash the bottle with phosphate buffered saline (PBS). Inoculate the culture with 105 TCID/mL of RABV strain ERA. Incubate the viral inoculum for 2 h under constant shaking at 37 °C in the presence of CO2 (5%) and under humid conditions.
- Remove the infection medium and add MEM supplemented with 5% (serum fetal bovine) SFB. Incubate for 72 h at 37 °C in the presence of CO2 (5%) in humid conditions.
- Harvest RABV 3 days after inoculation when the cells exhibit cytopathic effects. Freeze the infected cells and then thaw them at 4 °C to lyse the cells. Collect the medium from the bottle, and then place the cells into a centrifuge tube for centrifugation at 1,050 x g for 10 min at 4 °C.
- Store the supernatants from RABV at -80 °C, and prepare working aliquots for storage at -70 °C.
- Intracerebrally inoculate the virus obtained in the previous step into 21 day old female CD1 mice. Use a 1 mL syringe to inoculate 50 μL of solutions containing 106 MICLD50 (mouse infectious culture lethal dose 50%) doses into mice22.
4. RNA extraction
NOTE: Total RNA was extracted directly from bovine brains using an organic extraction method according to following protocol.
- Use 100 mg of brain tissue from each anatomical structure to extract total RNA using a commercially available reagent containing guanidium isothiocyanate.
- Manually homogenize a total of 100 mg of tissue from each structure in 1 mL of the guanidium isothiocyanate reagent by vortexing using a Dounce homogenizer with a small pestle inside the microtube for 15 s at maximum speed.
- Incubate the samples on ice for 5 min, and then add 200 μL of chloroform. Mix the samples vigorously for 15 s, incubate on ice for 2 to 3 min, and then centrifuge at 12,000 x g for 15 min at 4 °C.
- Transfer the aqueous phase to a clean tube and add 500 μL of isopropyl alcohol. Incubate the samples for 15 min at 21 °C.
- Centrifuge the samples at 12,000 x g for 10 min at 4 °C. Aspirate the aqueous supernatant by pipetting. The isolated RNA will be present as a pellet in the tube.
- Next, wash the RNA pellet with 1 mL of 75% ethanol by pipetting, and centrifuge at 7500 x g for 5 min at 4 °C.
- Decant the supernatant and air-dry the RNA pellet at room temperature (21 °C). Next, dilute the pellet in 50 μL of nuclease-free water.
- Determine the RNA concentration and quality at 260 nm using a spectrophotometer. Store RNA at -80 °C until use.
- Remove DNA from RNA samples by digestion with DNase I. Add 2 U of DNase I per 1 µg of RNA extracted in the previous step. Next, add 5 µL of 10x reaction buffer and then incubate at 37 °C for 30 min. Inactivate the DNase I by adding 1 μL of EDTA to the mixture followed by incubation for 10 min at 65 °C.
5. cDNA synthesis and PCR
- Synthesize first-strand cDNA using oligo dT and reverse transcriptase. For every 1 μg of RNA, add 1 μL of oligo DT (500 μg/mL), 1 μL of 10 mM dNTP mix, and nuclease-free distilled water to a final volume of 12 μL. Incubate the mixture at 65 °C for 5 min.
- Chill the reaction mixture to 4 °C. Centrifuge the tube for 30 s at 1,050 x g, and then add 4 μL of reaction buffer, 2 μL 0.1 M DTT, and 1 μL of ribonuclease inhibitor (40 U/μL).
- Incubate the reaction mixture at 37 °C for 2 min, and then add 1 µL of reverse transcriptase (200 U). Next, incubate the reaction mixture for 50 min at 37 °C, and then inactivate the reaction at 70 °C for 15 min.
- Store the cDNA at -20 °C prior to use.
NOTE: To verify the quality of the synthesized cDNA, perform the amplification of a 761 bp fragment of the RABV N gene prior to performing the synthesis in vitro. Use the protocol described by Loza-Rubio et al.11 as described in step 5.5 below. - Perform PCR using Taq DNA polymerase under the following conditions. Perform the reaction set up as follows: 10x Taq buffer containing 20 mM MgCl2, 0.2 mM dNTPs, 1 U Taq polymerase, 10 μM of each primer (SuEli+: 5′ cgtrgaycaatatgagtaca 3′ and SuEli-: 5′ caggctcraacattcttctta 3′), 2.5 μL of cDNA, and ultrapure water to a final volume of 50 µL.
- Perform the amplification in a thermocycler using the following cycling conditions: 95 ° C for 3 min; 35 cycles of 95 ° C for 30 s, 60 °C for 30 s, and 72 °C for 1 min; one cycle of 72 °C for 7 min.
- Assess the presence of the 761 bp PCR products using a 1% agarose gel.
6. In vitro transcription
NOTE: In vitro transcription generates mRNA of a target gene. Use a primer pair that amplifies the complete RABV N gene and one that is used as a positive control for the qRT-PCR assay. These primers are designed to amplify the complete RABV N gene and to add a promoter that recognizes the T7 polymerase (TAATACGACTCACTATAG).
- To amplify the whole N gene RABV, use the primers Trans-Nrab forward (5ʹ gatgagtcactcgaatatgtctt 3ʹ) and reverse (5ʹ caataatacgactcactatagggatggatgccgacaagatt 3ʹ) and a high-fidelity PCR kit.
- For each reaction, prepare a PCR master mix by adding to a clean PCR tube reaction buffer 10x, 2 mM DMSO, 25 mM MgCl2, 10 mM dNTPs, 10 μM of each primer, 0.5 U of high-fidelity polymerase, 1.5 μL of cDNA (prepared in step 5), and ultrapure water to a final volume of 50 µL.
- Use a thermocycler set to the following conditions for amplification: 94 °C for 1 min; 4 cycles of 94 °C for 1 min, 40 °C for 1 min, and 72 °C for 2 min; 35 cycles of 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 2 min; final extension of 72 °C for 2 min.
- To use the PCR product as a template for the in vitro synthesis and to remove components of the enzymatic reaction from step 6.1.2, purify the PCR product using a column-based concentrator kit according to the manufacturer's instructions, and then quantify using a spectrophotometer.
- For RNA synthesis, use an in vitro transcription kit with the following reaction mixture: 5 μL T7 Transcription 5x buffer, 1.9 μL of each triphosphate nucleotide, 10 μg of purified RABV N DNA VP2 DNA, and 2.5 μL of the enzyme mixture. Next, incubate the mixture at 37 °C for 4 h. Then, add 1 μL of 1 U/μg RNase-Free DNase to the reaction mixture and incubate for 15 min at 37 °C to eliminate DNA contamination.
- Purify the in vitro transcribed RNA and then concentrate it using phenol:chloroform:isoamyl alcohol (25:24:1).
- Resuspend the purified RNA pellet into 70 μL of TE buffer, and then store at -80 °C until use.
- Repeat the PCR protocol from step 6.1 until approximately 5 μg of PCR product is obtained. After purification and concentration, the in vitro transcribed N gene mRNA will be present at a concentration of 4.711 μg/μL.
- Confirm that the in vitro transcribed mRNA corresponds to the N gene following step 5 of this protocol and use this as a positive control for the real-time reverse transcription polymerase chain reaction (qRT-PCR).
7. Real-time reverse transcription polymerase chain reaction (qRT-PCR)
- For qRT-PCR, use an in vitro mRNA transcript encoding the complete N gene as a positive control along with a commercial one-step qRT-PCR kit using hybridization probes according to the manufacturer's instructions. Use the probe and primers described by Carneiro et al. (2010)23 to detect a conserved region of the RABV gene N (BRDesrot-Rev: 5′ aaactcaagagaaggccaacca 3′, BRDesrot-Fwd: 5′ cgtactgatgtggaagggaattg 3′, and BRDesrot-Probe: 5′-FAM-acaagggaccctactgtttcagagcatgc-3′-BHQ).
- Use the following thermal cycling conditions: 1 cycle of 50 °C for 10 min and 95 °C for 2 min; 40 cycles of 95 °C for 15 s and 50 °C for 30 s.
8. Calibration curve or standard curve
- Perform serial dilutions of the in vitro transcribed mRNA by serially pipetting 1 μg/μL of mRNA into tubes until six decuple dilutions are obtained. Prepare tubes at a 100 μL volume in triplicate for use in creating the standard curve. Perform qRT-PCR as described in step 7.
- Perform qRT-PCR in a thermocycler with a rotor by processing the various brain samples simultaneously with the running reactions for creating the standard curve and using positive controls, negative controls, and supernatants isolated from cell culture.
- To evaluate the limit of detection, test efficacy, and sensitivity of the test, use a standard curve in triplicate under the same conditions.
9. qRT-PCR of biological samples
- For the detection of RABV gene N, use the RNA from field samples, positive controls, negative controls, and cell culture supernatants to perform qRT-PCR using the PCR kit described in step 7.
- To determine the number of copies of the RABV genome present in the bovine brain samples, obtain Ct data from the standard curve and biological samples and from those interpolated from the calibration curve equation.
Representative Results
DFA results showed 100%, 100%, 83.3%, 66%, and 50% positivity for RABV in the cortex, thalamus, medulla, pons, and horn, respectively. These results confirmed the previous results, and at least three of the structures dissected from each brain were positive for RABV. A representative positive DAF staining is shown in Figure 1.
Figure 2 shows the amplification of a fragment of the RABV N gene (step 5.5) with the primers first ones reported by Loza-Rubio et al.11. This showed that the material obtained to be used in amplification was of good quality.
The size of the PCR-amplified fragment is shown in Figure 3. The equation of the standard curve shows the relationship between the amount of RABV genetic content in a sample and the size of the PCR-amplified fragment. The amount of RABV genetic content (in ng) was deduced by interpolation of the Ct values in the calibration curve using the equation presented in Figure 4. Using this equation, the detection limit of 1 x 105 μg/μL of viral RNA was found to correspond to 36 copies of the RABV genome. These results demonstrate that the assay is sensitive and can be used to detect the virus in samples that contain a low number of copies RABV N gene. The efficiency of the assay was calculated using the slope of the standard curve, which was -3.28. The samples used for qRT-PCR showed amplification of the RABV N gene.
To determine the number of viral copy present, the Ct values for each sample were interpolated using the calibration curve equation. The result obtained (in nanograms) was substituted into the formula described below from the following ref.24:
Number of copies RABV N gene = (ng sample * 6.022 x 1023) / (104 bp (length of PCR product) * 1 x 109 * 650).
Comparatively, the results of qRT-PCR assays were consistent with those obtained using DFA. According to the results obtained in the qRT-PCR test in cases C and D (Table 1), the thalamus is the structure that possesses the highest number of copies of the RABV N gene. This suggests that early infection of hypothalamic and thalamic neurons is important in the development of the disease, given that these neurons control the vegetative functions of an animal. The copy numbers of the RABV N gene that were detected in each structure of each sample are shown in Table 1. The sensitivity and specificity of the qRT-PCR test were both 100%, as all samples were positive. This result is consistent with the initial diagnoses of the samples.
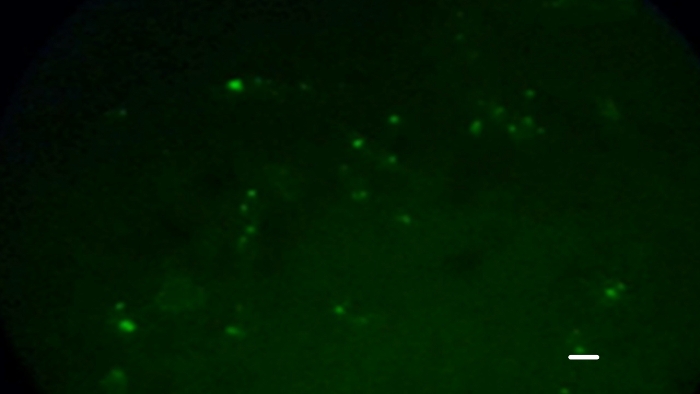
Figure 1: Bovine brain tissues showing positive staining according to the direct immunofluorescence test that detects rabies virus protein N in infected tissues. An apple green coloration is observed upon staining, where the antibody binds to the rabies antigen. Scale bar: 50 µm Please click here to view a larger version of this figure.
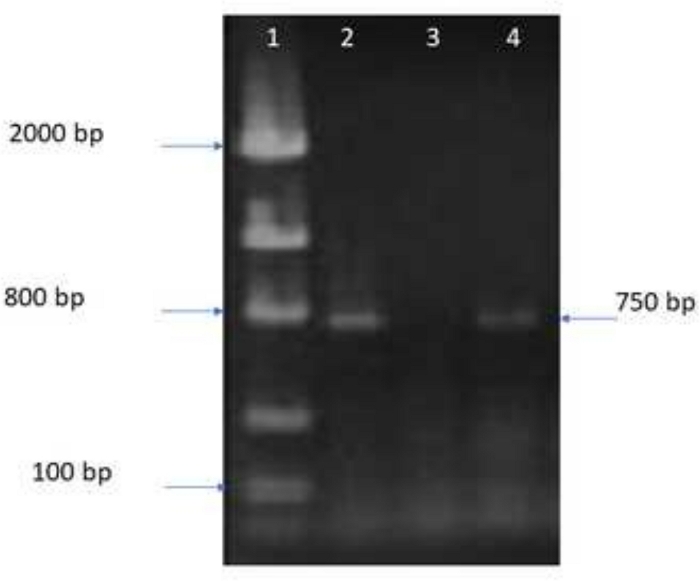
Figure 2: 1.5% agarose gel showing the amplification products. Amplification of a 761 bp fragment of the RABV N gene to demonstrate the presence and quality of the cDNA to be used in in vitro transcription. Lane 1 contains a molecular weight marker, line 2: amplification of positive control; lane 3: negative control (non-infected sample); line 4: amplification of cDNA used for in vitro transcription. Please click here to view a larger version of this figure.
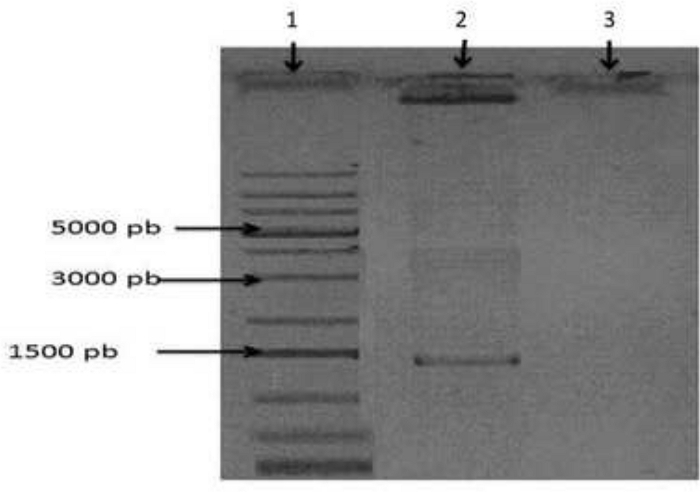
Figure 3: 1.5% agarose gel showing the amplification products. Amplification of the complete N gene is shown in lane 2. Lane 1 contains a molecular weight marker, and lane 3 contains the negative amplification control. Please click here to view a larger version of this figure.
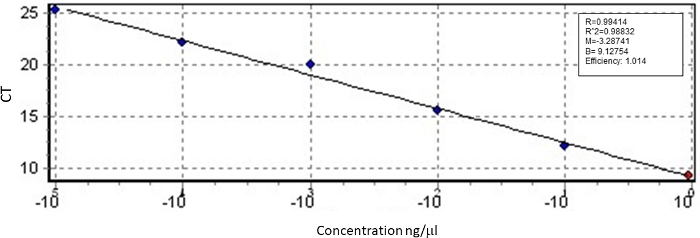
Figure 4: Standard curve of the qRT-PCR using in vitro transcribed N gene mRNA to quantify the number of copies of the RABV N gene. Please click here to view a larger version of this figure.
| ID brain | Structure | DAF | Number copies (x109) |
| A | Ammon’s horn | + | 0.261 |
| Cerebellum | + | 0.0663 | |
| Cortex | + | 0.02 | |
| Medulla | + | 0.146 | |
| Pons | + | 30.7 | |
| Thalamus | + | 108 | |
| B | Ammon’s horn | – | 0.0251 |
| Cerebellum | + | 4.64 | |
| Cortex | + | 12.4 | |
| Medulla | + | 0.175 | |
| Pons | ‒ | 0.0721 | |
| Thalamus | + | 121 | |
| C | Ammon’s horn | ‒ | 0.168 |
| Cerebellum | + | 0.0239 | |
| Cortex | + | 21.5 | |
| Medulla | + | 17.2 | |
| Pons | + | 1.32 | |
| Thalamus | + | 102 | |
| D | Ammon’s horn | + | 11.8 |
| Cerebellum | + | 0.0239 | |
| Cortex | + | 8.72 | |
| Medulla | + | 1.25 | |
| Pons | ‒ | 0.0165 | |
| Thalamus | + | 33.4 | |
| E | Ammon’s horn | + | 4.15 |
| Cerebellum | + | 6.78 | |
| Cortex | + | 1.53 | |
| Medulla | + | 1.41 | |
| Pons | + | 0.025 | |
| Thalamus | + | 95 | |
| F | Ammon’s horn | + | 0.0221 |
| Cerebellum | ‒ | 0.00023 | |
| Cortex | + | 4.95 | |
| Medulla | ‒ | 0.000556 | |
| Pons | + | 1.02 | |
| Thalamus | + | 89 |
Table 1: Determination of the number of copies of the RABV N gene within each brain structure. Results were obtained from six anatomical structures of RABV-positive bovine brains diagnosed using DFA. The words highlighted in bold indicate the structures with the most copies. The results were expressed as number of copies of the N gene per milligram of tissue.
Discussion
Previous studies have shown that DFA can only detect RABV within seven days of the sample being stored at room temperature (21 °C)14. In contrast, this work demonstrated that the sensitivity of RT-PCR begins to decrease after the samples have been exposed to room temperature for 12 days. Therefore, the RABV genome can be detected by qRT-PCR in samples exposed to room temperature for up to 23 days. This demonstrates that the sensitivity of qRT-PCR is relatively higher for more highly decomposed samples. However, it remains necessary to develop simpler methods that do not compromise specificity25. The present research demonstrated a molecular assay that possesses higher sensitivity than DFA, based on the observation that it was able to detect the viral genome regardless of the specific brain structure.
The advantages of the RT-PCR technique for the use in detecting infectious agents have been highlighted in several scientific studies. However, this technique can exhibit some disadvantages, such as cost of execution, as it requires specialized equipment. Trained personnel are required to handle molecular biology assays. Additionally, it should be mentioned that as a highly sensitive technique, some of the steps are critical to obtain the best results. One of these is the proper handling of samples for RNA extraction. This step is crucial, as RNA is easily degraded, and the results could, therefore, be affected. Consider maintaining the sample in cold conditions (approximately 4 °C) during the process. In addition, consider that several rounds of PCR application are carried out as mentioned in step 6.1 to generate the transcript in vitro. This is because a large amount of genetic material is necessary for the reaction to yield substantial (more than milligram) quantities of DNA. Another crucial aspect of this assay is the requirement for purification of the genetic material in the columns, as DNA may also be lost during this step.
qRT-PCR is known to be as specific as the DFA test, which is the standard test approved by the WHO and the OIE. However, molecular assays employing RT-qPCR have been shown to have a higher sensitivity and to thus allow for analysis of samples with a higher degree of decomposition to facilitate the detection of a relatively small number of copies of the viral genome. It is also much faster, reduces the risk of contamination, and can be used ante-mortem26,27.
Bingham and van Der Merwe (2002)28 conducted a study using DFA to determine which brain structures possessed higher concentrations of the RABV N gene. A total of 252 brain structures from several species were evaluated. The thalamus, pons, and medulla were the most relevant brain structures, as they were positive in all evaluated brain samples. These results agree with the results presented here, which were consistent with DFA results in the following structures: cortex and thalamus, 100%; medulla, 83.3%; pons, 66%; and horn, 50%. In contrast, previous studies reported no false negatives. In this work, some negative results were detected in complete brain structures that had previously been diagnosed as positive. This may be because the portion of the sample used for the test did not contain viral particles identified by the antibody or the more sensitive molecular assay. Another possible explanation is that the samples were not properly transported or stored prior to reception at the laboratory.
The qRT-PCR assay conducted in this study could detect as few as 36.3 copies of the RABV N gene per milligram of tissue. The most suitable brain structures for qRT-PCR included the cortex and thalamus. This is based on the observations that higher concentrations of the RABV N gene were detected in these structures and that they were also found to be positive using the RABV reference test. The test was able to detect very low concentrations (0.00023 x 109 copies) of the virus in various samples. In addition to quantifying the viral particles in the sample, the test appears to provide a good alternative diagnostic and research tool for the detection of RABV.
With the results obtained using the rabies virus viral genome detection protocol presented here, it is anticipated that this technique can be applied for the molecular diagnosis of the virus in different types of samples, as it is capable of detecting minimal amounts of the viral genome. Its biggest advantage over other methods such as DFA is that it is more sensitive and can be used ante-mortem, as has been suggested by other authors.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institute of Agricultural, Forestry, and Livestock Research (INIFAP). We thank Jerzayn Fraustro Esquivel for his collaboration in the development of the video associated with this document.
Materials
| Chloroform | SIGMA | C7559 | Facilitates recovery of the aqueous phase of PCRs which have been overlaid with mineral oil. |
| DNA Clean & Concentrator-500 | Zimo | D4031 | The DNA Clean & Concentrator-500 (DCC-500) is designed for the rapid, large format purification and concentration of up to 500 µg of high quality DNA from samples including large-scale restriction endonuclease digestions and impure DNA preparations. |
| Ethanol | Amresco | 193-500 | Purification of nucleic acids |
| FastStart High Fidelity PCR System kit, dNTPack | Roche | 3553400001 | High fidelity enzyme for the amplification of PCR products avoiding random base changes |
| GelDoc XR | BioRad | XR+ | Analyzes larger protein and DNA gels |
| GelRed | Biotium | 41003 | A new generation of nucleic acid gel stains, they possess novel chemical features designed to minimize the chance for the dyes to interact with nucleic acids in living cells. |
| iCycler Thermal Cycler Gradient | BioRad | 582BR | Temperature can be monitored and controlled by instrument algorithm, in-sample probe, or sample block modes |
| iTaq Universal Probes One-Step Kit | BioRad | 1725141 | Reaction mixture to carry out PCR reactions in real time using TaqMan type hybridization probes |
| Isopropyl alcohol | Amresco | 0918-500 | Precipitation of nucleic acids |
| QIAquick gel extraction kit | Qiagen | 28706 | QIAquick Kits contain a silica membrane assembly for binding of DNA in high-salt buffer and elution with low-salt buffer or water. The purification procedure removes primers, nucleotides, enzymes, mineral oil, salts, agarose, ethidium bromide, and other impurities from DNA samples |
| M-MLV Reverse Transcriptase | Invitrogen | 28025-021 | Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT) uses singlestranded RNA or DNA in the presence of a primer to synthesize a complementary DNA strand. |
| NanoDrop 2000 | Thermo-Scientific | ND2000 | Microvolume Spectrophotometer |
| Oligo(dT)18 primer | Invitrogen | SO132 | The oligo (dT)18 primer is a synthetic single-stranded 18-mer oligonucleotide with 5'- and 3'-hydroxyl ends. |
| RiboMAX Large Scale RNA Production Systems kit SP6 and T7 | Promega | P1300 | In vitro transcription reactions are used to synthesize microgram amounts of RNA probes from recombinant DNA templates. Most transcription reactions designed to generate RNA probes are optimized to maximize incorporation of radiolabeled ribonucleotides rather than to produce large amounts of RNA |
| Taq DNA Polymerase | Invitrogen | #EP0402 | Taq DNA Polymerase is a highly thermostable DNA polymerase of the thermophilic bacterium Thermus aquaticus. The enzyme catalyzes 5’ and 3’ synthesis of DNA |
| TRIzol reagent | Invitrogen | 15596026 | The TRIzol reagent is a complete, ready-to-use reagent, designed for the isolation of high quality total RNA or the simultaneous isolation of RNA, DNA and proteins from a variety of biological samples. |
References
- Subramaniam, M. R., Madhusudana, S. Laboratory diagnosis of human rabies: recent advances. ScientificWorld Journal. 2013 (569712), 1-10 (2013).
- . CDC Available from: https://www.cdc.gov/rabies/diagnosis/animals-humans.html (2019)
- Dean, D. J., Abelseth, M. K., Atanasiu, W., Meslin, F. X., Kaplan, M. M., Koprowski, H. The fluorescent antibody technique in rabies. Laboratory techniques in rabies. , (1996).
- Prabhu, K. N., et al. Application and comparative evaluation of fluorescent antibody, immunohistochemistry, and reverse transcription polymerase chain reaction tests for the detection of rabies virus antigen or nucleic acid in brain samples of animals suspected of rabies in India. Veterinary Science. 5 (1), 24 (2018).
- Woldehiwet, Z. Clinical laboratory advances in the detection of rabies virus. Clinica Chimica Acta. 351 (1-2), 49-63 (2005).
- Singh, R., et al. Rabies – epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: a comprehensive review. Veterinary Quarterly. 37 (1), 212-251 (2017).
- David, D. Role of the RT-PCR method in ante-mortem & post-mortem rabies diagnosis. Indian Journal of Medical Research. 135 (6), 809-811 (2012).
- Biswal, M., Ratho, R. K., Mishra, B. Role of reverse transcriptase polymerase chain reaction for the diagnosis of human rabies. Indian Journal of Medical Research. 135, 837-842 (2012).
- Wacharapluesadee, S., et al. Comparative detection of rabies RNA by NASBA, real-time PCR and conventional PCR. Journal of Virological Methods. 175 (2), 278-282 (2011).
- Mani, R. S., et al. Utility of real-time Taqman PCR for ante mortem and post-mortem diagnosis of human rabies. Journal of Medical Virology. 86 (10), 1804-1812 (2014).
- Loza-Rubio, E., Rojas-Anaya, E., Banda-Ruiz, V. M., Nadin-Davis, S. A., Cortez-Garcia, B. Detection of multiple strains of rabies virus RNA using primers designed to target Mexican vampire bat variants. Epidemiology and Infection. 133 (5), 927-934 (2005).
- Dedkov, V. G., et al. Development and evaluation of a RT-qPCR assay for fast and sensitive rabies diagnosis. Diagnostic Microbiology and Infectious Disease. 90, 18-25 (2018).
- Boldbaatar, B., et al. Rapid detection of rabies virus by reverse transcription loop-mediated isothermal amplification. Japanese Journal Infectious Diseases. 62, 187-191 (2009).
- Rojas Anaya, E., Loza Rubio, E., Banda Ruiz, V., Hernández-Baumgarten, E. Use of reverse transcription-polymerase chain reaction to determine the stability of rabies virus genome in brains kept at room temperature. Journal of Veterinary Diagnostic Investigation. 18 (1), 98-101 (2006).
- Dacheux, L. Dual combined Real-Time reverse transcription polymerase chain reaction assay for the diagnosis of Lyssavirus infection. PLoS Neglected Tropical Diseases. 10 (7), 004812 (2016).
- Tamay De Dios, L., Ibarra, C., Velasquillo, C. Fundamentos de la reacción en cadena de la polimerasa (PCR) y de la PCR en tiempo real. Investigigación en Discapacidad. 2 (2), 70-78 (2013).
- Chapter 2.1.17. Rabies (infection with rabies virus and other Lyssaviruses. OIE Available from: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.17_RABIES.p (2018)
- Wacharapluesadee, S., Hemchudha, T. Ante-and post-mortem diagnosis of rabies using nucleic acid-amplification tests. Expert Reviews of Molecular Diagnostics. 10 (2), 207-218 (2010).
- Protocol for Postmortem Diagnosis of Rabies in Animals by Direct Fluorescent Antibody Testing. CDC Available from: https://www.cdc.gov/rabies/pdf/rabiesdfaspv2.pdf (2017)
- Dean, D. J., Abelseth, M. K., Atanasiu, W., Meslin, F. X., Kaplan, M. M., Koprowski, H. The fluorescent antibody technique in rabies. Laboratory techniques in rabies. , (1996).
- Cuevas-Romero, S., Colmenares, V. G., Batalla, C. D., Hernández, B. E. Selección de un virus rábico de origen vampiro para utilizarse como cepa de desafío en bovino. Veterinaria México. 20, 271-275 (1989).
- Mendez-Ojeda, M. L., et al. Detection of rabies virus in organs unrelated to the central nervous system of experimentally inoculated vampire bats. Revista Méxicana de Ciencias Pecuarias. 9 (3), 435-450 (2018).
- Carneiro, A. J., et al. Rabies virus RNA in naturally infected vampire bats, Northeastern Brazil. Emerging Infectious Diseases. 16, 2004-2006 (2010).
- Beltran, F. J., Dohmen, F. G., Del Pietro, H., Cisterna, D. M. Diagnosis and molecular typing of rabies virus in samples stored in inadequate conditions. The Journal of Infection in Developing Countries. 13 (8), 1016-1021 (2018).
- Finke, S., Cox, J. H. Differential transcription attenuation of rabies virus genes by intergenic regions: generation of recombinant viruses overexpressing the polymerase gene. Journal of Virology. 74 (16), 7261-7269 (2000).
- Fooks, A. R., et al. . Rabies. 3, 1-18 (2017).
- Bingham, J., Van Der, M. M. Distribution of rabies antigen in infected brain material: determining the reliability of different regions of the brain for the rabies fluorescent antibody test. Journal Virological Methods. 101 (1-2), 85-94 (2002).

